Oxidation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Oxidation Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Good morning everyone! Today, we're going to explore oxidation reactions specifically focusing on carbon compounds. To start, can anyone tell me what oxidation generally involves?

Isn’t it when a substance gains oxygen or loses electrons?

Exactly! Oxidation involves gaining oxygen. It's a critical concept in chemistry as it is often involved in energy production and metabolic processes.

How does this apply to carbon compounds?

Great question! Carbon compounds, especially alcohols, can undergo oxidation. For example, ethanol can be oxidized to become ethanoic acid.

What substances do we use for this oxidation?

We use oxidizing agents like alkaline potassium permanganate or acidified potassium dichromate. They're effective for converting alcohols to acids.

Remember the acronym *OXIDATE* for oxidation: *Oxygen eXchange Identifies the Decrease of Electrons*.
Real-life Application of Oxidation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the basics, let’s consider the practical implications. Can anyone think of a real-life example of oxidation?

I believe alcohol turning into vinegar is one of them!

Absolutely! When ethanol is oxidized, it forms ethanoic acid, which is vinegar. This process is utilized in both medicinal and culinary applications.

What about the role of potassium permanganate in this process?

Excellent query! Potassium permanganate acts as the oxidizing agent in the reaction. Initially, its purple color disappears as it reacts with ethanol.

Does that reaction happen with any alcohol?

Good follow-up! While many alcohols can oxidize, they may not all yield acids. Each alcohol has unique properties, influencing its oxidation pathway.

To remember the role of oxidizing agents, think *OX-MAN* for *Oxidizing Agents Converting Alcohols*.
Experiments with Oxidation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s conduct an experiment. We’ll heat ethanol in a water bath and add potassium permanganate. Can anyone predict what will happen?

The purple color should fade, right?

Correct! The disappearance of color indicates that oxidation is occurring. Ethanol transforms into ethanoic acid.

What if I add more permanganate after the color disappears?

Great observation! If excess permanganate is added, the color will remain unchanged. This indicates that all ethanol has been oxidized.

So, it’s a visual confirmation of the reaction?

Exactly! Visual indicators are crucial in chemistry. You can remember the phrase *OXIDATION REVEAL* to link oxidation to indicators in reactions.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we delve into oxidation reactions as they pertain to carbon compounds. It discusses how certain substances can add oxygen to others, highlighting the role of oxidizing agents, and presents a classic example of the oxidation of ethanol to ethanoic acid using potassium permanganate.
Detailed
Oxidation in Carbon Compounds
Oxidation reactions are significant processes in chemistry where substances add oxygen or lose electrons. In the context of carbon compounds, this section focuses predominantly on how alcohols can be oxidized to carboxylic acids. Alcohols like ethanol can undergo oxidation when subjected to oxidizing agents such as alkaline potassium permanganate or acidified potassium dichromate.
Key Points Covered:
- Oxidation Reactions: Oxidation is characterized by the addition of oxygen to a substance and the removal of hydrogen or electrons.
- Ethanol to Ethanoic Acid: A practical demonstration involves adding a solution of alkaline potassium permanganate to ethanol, where its purple color dissipates, indicating the oxidation process as ethanol transitions to ethanoic acid.
- Oxidizing Agents: Substances capable of facilitating oxidation include alkaline potassium permanganate, which serves as an effective oxidizing agent during the reaction.
This phenomenon showcases the foundational principle that alcohols are convertible to acids through oxidation, which is essential for a variety of biological and industrial applications.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Oxidation in Carbon Compounds
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
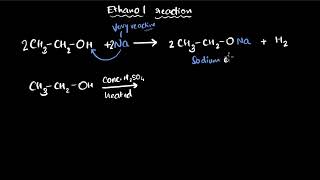
You have learnt about oxidation reactions in the first Chapter. Carbon compounds can be easily oxidised on combustion. In addition to this complete oxidation, we have reactions in which alcohols are converted to carboxylic acids.
Detailed Explanation
In chemistry, oxidation refers to the process where a substance loses electrons or gains oxygen. In this context, carbon compounds, when burned (combustion), undergo oxidation. For instance, when ethanol, which is a type of alcohol, is completely oxidised, it gets converted to acetic acid (a carboxylic acid). This shows that carbon compounds are versatile and can undergo transformations during reactions.
Examples & Analogies
Think of oxidation like cooking food. When you cook or burn something, it changes its form completely, just like how vinegar (acetic acid) is produced from ethanol when it is oxidised. Just like how charred food indicates it has been overcooked, the products produced after oxidation give clues about the process that took place.
Oxidising Agents
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We see that some substances are capable of adding oxygen to others. These substances are known as oxidising agents. Alkaline potassium permanganate or acidified potassium dichromate are oxidising agents used to oxidise alcohols to acids, that is, adding oxygen to the starting material.
Detailed Explanation
Oxidising agents are substances that can accept electrons from other substances, thereby enabling oxidation reactions. For example, alkaline potassium permanganate and acidified potassium dichromate can add oxygen to alcohols, converting them into acids. This is important in many chemical reactions, especially in organic chemistry, where the conversion of compounds to others with different properties is often required.
Examples & Analogies
Imagine oxidising agents as sparks in a fire. Just as a spark can ignite a wood log and trigger a big fire, oxidising agents kickstart chemical reactions that change substances from one form to another. For instance, adding a few drops of an oxidising agent to ethanol can lead to the production of acetic acid, much like how a small spark can lead to a large flame.
Experimental Observation with Potassium Permanganate
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Take about 3 mL of ethanol in a test tube and warm it gently in a water bath. Add a 5% solution of alkaline potassium permanganate drop by drop to this solution. Does the colour of potassium permanganate persist when it is added initially? Why does the colour of potassium permanganate not disappear when excess is added?
Detailed Explanation
In this experiment, the aim is to observe how potassium permanganate behaves when added to ethanol. When potassium permanganate is first added, it has a deep purple color, which disappears as it reacts with the ethanol. If the reaction goes to completion and there is no ethanol left, adding excess potassium permanganate doesn't change the solution further, which causes the color to persist. This indicates whether all the ethanol has reacted or if there is still some left.
Examples & Analogies
Think of this experiment like adding food dye to a glass of water. As long as there is clear water, the dye will spread throughout. But if you keep adding dye after the water is fully colored, the water remains dyed and you won't see any change. Similarly, ethanol initially consumes the potassium permanganate, but if it's all used up, you'll see the color remain when excess dye (oxidising agent) is added.
Key Concepts
-
Oxidation: The process is crucial in chemistry where substances gain oxygen or lose electrons.
-
Oxidizing Agents: Substances that facilitate the oxidation of other compounds by providing oxygen.
-
Ethanol to Ethanoic Acid: A common example of oxidation where ethanol transforms into ethanoic acid.
-
Visual Indicators: The color change of potassium permanganate acts as a visual cue for oxidation.
Examples & Applications
The oxidation of ethanol (C₂H₅OH) to ethanoic acid (CH₃COOH) using potassium permanganate.
Combustion reactions of carbon compounds releasing energy.
The use of oxidizing agents in organic chemistry to convert alcohols into carboxylic acids.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the reaction where ethanol's sweet, Turns to acetic acid, oh what a treat!
Stories
Imagine a chef named Ethan who loves cooking with alcohol. One day, he adds a magical purple potion to his brew. Poof! The drink transforms into a tangy vinegar he uses in his salads!
Memory Tools
Think of the word EAT - Ethanol Added Transforms for remembering how ethanol can turn into ethanoic acid.
Acronyms
Use the acronym *AGENT* for
*A*dd *G*ain *E*lectrons *N*eeded for *T*ransformation*.
Flash Cards
Glossary
- Oxidation
A chemical process where a substance gains oxygen or loses electrons.
- Oxidizing Agent
A substance that causes oxidation by providing oxygen or removing electrons.
- Ethanol
An alcohol with the chemical formula C₂H₅OH, used as a solvent and in beverages.
- Ethanoic Acid
Also known as acetic acid; a carboxylic acid formed by the oxidation of ethanol.
- Potassium Permanganate
An oxidizing agent used in chemical reactions, known for its deep purple color.
Reference links
Supplementary resources to enhance your learning experience.
