Will you be my Friend?
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
The Versatility of Carbon
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore the concept of how carbon is a very versatile element, forming bonds with various other elements.

Why is carbon considered versatile?

Great question! Carbon can form covalent bonds with different elements like oxygen, nitrogen, halogens, and sulfur, resulting in a wide variety of compounds. We can remember this with the mnemonic 'C-O-N-S-H' - Carbon, Oxygen, Nitrogen, Sulfur, and Hydrogen.

What do you mean by functional groups?

Functional groups are specific groupings of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. For example, the hydroxyl group -OH in alcohols.

Can the same carbon chain have different functional groups?

Exactly! And these changes in functional groups can alter the properties of the carbon compound despite having the same carbon skeleton.

So, can you give an example of how these functional groups affect properties?

Certainly! Consider alcohols - the presence of the hydroxyl group makes compounds like ethanol soluble in water, while hydrocarbons without this group are typically hydrophobic.

To summarize, carbon's ability to bond with various elements and form diverse functional groups is crucial for creating the vast array of organic compounds we see around us.
Heteroatoms and Their Role
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's focus on heteroatoms. Can anyone tell me what a heteroatom is?

Is it an atom that is not carbon or hydrogen in a compound?

Exactly! Heteroatoms are atoms like oxygen or nitrogen that can replace hydrogen in hydrocarbons. This replacement allows for the formation of functional groups.

So, would an alcohol be a hydrocarbon with a hydroxyl group?

Yes! An alcohol is a hydrocarbon in which a -OH group replaces one hydrogen atom. This change significantly alters the compound's properties, giving it polar characteristics.

What about when carbon bonds with halogens? How does that work?

Great inquiry! When carbon bonds with halogens, it leads to the formation of haloalkanes, which are significant in organic chemistry due to their reactivity and wide applications.

So, in summary, heteroatoms replace hydrogen in carbon compounds to form functional groups, which significantly influence the chemical behavior of those compounds.
Homologous Series
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s talk about homologous series. Who can explain what it is?

A series of compounds that have similar chemical properties?

Correct! A homologous series consists of compounds that have the same functional group and differ by a -CH2- unit. For instance, in the case of alcohols like methanol and ethanol, they show similar properties.

Does the physical properties change in these series?

Yes, as you increase the number of carbon atoms, the melting and boiling points increase due to the larger molecular size.

That's interesting! So, what's the general formula for alkenes in the homologous series?

The general formula for alkenes is CnH2n, where 'n' is the number of carbon atoms.

In summary, homologous series allow us to predict the behavior of new compounds based on the properties of existing compounds with similar functional groups.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section discusses how carbon forms a wide array of compounds by bonding with different elements, emphasizing the role of heteroatoms and functional groups in determining properties. The text also introduces the concept of homologous series, demonstrating how similar functional groups influence the behavior of various carbon chains.
Detailed
In this section, we delve into the versatile nature of carbon and its ability to form numerous bonds with other elements such as halogens, oxygen, nitrogen, and sulfur. This section highlights the importance of heteroatoms in replacing hydrogen in hydrocarbon chains while still satisfying carbon's tetravalency. Functional groups, which confer specific properties to compounds, are crucial to understanding organic chemistry. The concept of homologous series is introduced, where groups of compounds with similar structure but varying lengths show gradual changes in physical properties while maintaining similar chemical properties due to their functional groups. This versatility of carbon, with its property of catenation and tetravalency, underpins the multitude of organic compounds present in nature.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
The Versatile Friend: Carbon’s Reactions
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Carbon seems to be a very friendly element. So far we have been looking at compounds containing carbon and hydrogen only. But carbon also forms bonds with other elements such as halogens, oxygen, nitrogen and sulphur.
Detailed Explanation
This chunk highlights carbon's ability to bond not just with hydrogen but also with various other elements, which expands the variety of compounds it can form. This property is important because these bonds create different substances that have distinct characteristics and uses. By forming these relationships with elements like halogens (like chlorine or bromine), oxygen, nitrogen, and sulfur, carbon can create a range of organic molecules that serve multiple purposes across biological and industrial fields.
Examples & Analogies
Think of carbon as a versatile friend in a group who can hang out with different cliques. Just like how this friend adapts their behavior to fit in with different groups, carbon changes its bonding patterns to create everything from simple hydrocarbons, like methane, to complex compounds, like amino acids in proteins.
Heteroatoms and Functional Groups
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
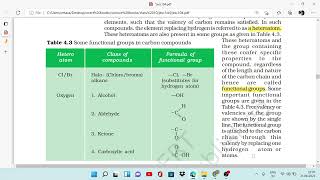
In a hydrocarbon chain, one or more hydrogens can be replaced by these elements, such that the valency of carbon remains satisfied. In such compounds, the element replacing hydrogen is referred to as a heteroatom. These heteroatoms are also present in some groups as given in Table 4.3.
Detailed Explanation
The concept of heteroatoms is crucial for understanding how carbon compounds can gain unique properties. When hydrogens in a carbon compound are replaced by these heteroatoms (like chlorine or oxygen), they create functional groups. These functional groups define the chemical behavior of the molecules, affecting reactivity, polarity, and even how the substance smells or tastes. For instance, replacing a hydrogen with a hydroxyl group (-OH) turns an alkane (like ethane) into an alcohol (like ethanol), increasing its solubility in water and changing its reactivity.
Examples & Analogies
Imagine you're in a cooking class where the main ingredient is rice (hydrocarbons). When you add different spices (heteroatoms) like salt (sodium), lemon (oxygen), or chili (halogens), each dish becomes distinct. Just like these spices change the flavor and appeal of the rice, heteroatoms transform carbon compounds into something new and useful.
Functional Groups and Their Importance
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
These heteroatoms and the group containing these confer specific properties to the compound, regardless of the length and nature of the carbon chain, and hence are called functional groups.
Detailed Explanation
Functional groups are a cornerstone in organic chemistry because they dictate the reactivity and the characteristics of carbon compounds. For example, the presence of a carboxyl group (-COOH) turns a hydrocarbon into a carboxylic acid, which is typically more reactive than its hydrocarbon counterpart. These functional groups are like instructions that control how a molecule behaves in a chemical reaction, influencing everything from acidity to how the molecule interacts with other substances in the environment.
Examples & Analogies
Think of functional groups as keys on a keyboard. Each key (functional group) provides different inputs (properties and reactions) based on the typed commands (reactions the compound can take part in). Just as different commands lead to different outcomes on a computer, the presence or absence of these functional groups leads to wildly different properties and behaviors in chemical reactions.
Combining With Heteroatoms
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Some important functional groups are given in Table 4.3.
Detailed Explanation
This chunk suggests that different functional groups can be classified based on the heteroatoms present. Each group changes the behavior of the carbon compound. For instance, alcohols have hydroxyl groups (-OH), while amino acids contain amine functional groups (-NH2). Knowing these classifications is crucial for students to make sense of organic reactions and synthesizing new compounds.
Examples & Analogies
Imagine cooking with different utensils: a pot does a great job for boiling, but a frying pan is better for sautéing. Similarly, each functional group works best for specific reactions — alcohols might dissolve better in certain solvents than acids do. Understanding which utensil to use, or which functional group to work with, is essential for achieving the desired results in both cooking and chemistry.
Key Concepts
-
Heteroatoms: Non-carbon atoms in organic molecules that replace hydrogen.
-
Functional Groups: Specific groups that determine the chemical properties of compounds.
-
Homologous Series: Groups of organic compounds with similar chemical properties differing by a -CH2- unit.
Examples & Applications
The formation of ethanol from ethene illustrates how functional groups affect compounds.
Methanol, ethanol, and propanol demonstrate a homologous series where each compound differs by a -CH2- unit.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Carbon bonds with C-O-N-S, providing compounds that are a chemical fest!
Stories
Imagine a friendly carbon inviting its friends like oxygen and nitrogen to create a diverse party of compounds.
Memory Tools
HC=HALO (in hydrocarbon) - H, C, and the essential functional groups like Hydroxyl.
Acronyms
HFG - Heteroatoms, Functional groups, and the importance of the Homologous series!
Flash Cards
Glossary
- Heteroatom
An atom in a compound that is not carbon or hydrogen.
- Functional Group
A specific group of atoms within a molecule that are responsible for its characteristic reactions.
- Homologous Series
A series of compounds with the same functional group and differences in molecular mass or structure.
Reference links
Supplementary resources to enhance your learning experience.
