Saturated and Unsaturated Carbon Compounds
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Organic Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore organic compounds derived from carbon. Why do you think carbon is so significant in forming these compounds?

I think it's because carbon can bond with many other elements.

Exactly! Carbon's ability to form four bonds allows it to create diverse structures including chains and branches. This versatility leads to the formation of millions of organic compounds.

What do you mean by saturated and unsaturated compounds?

Good question! Saturated compounds contain only single bonds, while unsaturated compounds include double or triple bonds. This affects their reactivity and physical properties.

Can you give examples of each type?

Sure! Methane (CH₄) is a saturated hydrocarbon, while ethene (C₂H₄) is an unsaturated hydrocarbon.

So, is all life based on carbon?

Yes! Every living organism relies on carbon compounds. This makes understanding carbon essential for biology and medicine.

In summary, carbon's tetravalency and the ability to catenate are foundational for the vast diversity of organic compounds.
Properties of Carbon Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about some properties of carbon. What do you think are key characteristics that allow carbon to form so many compounds?

Maybe its size and how it can share electrons?

That's right! Carbon's small size enables strong bonds with other atoms while its four valence electrons allow extensive sharing. This is crucial in forming covalent bonds.

What about catenation?

Catenation is the property of carbon to bond with itself, resulting in chains and rings. This property is unique to carbon compared to other elements.

And this leads to structural isomers?

Exactly! Different arrangements of the same atoms lead to various organic compounds with unique properties.

Can you give an example of a structural isomer?

Sure! Butane (C₄H₁₀) can exist as a straight-chain version and a branched version. Both have the same molecular formula, but different structures.

To recap, carbon's tetravalency, catenation, and ability to form stable compounds make it the backbone of organic chemistry.
The Role of Functional Groups
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's dive into functional groups. Why do you think they are important in organic compounds?

They probably determine how a compound behaves chemically.

Correct! Functional groups restrict the physical and chemical properties of compounds. For example, alcohols contain an -OH group which makes them polar and hydrophilic.

Can you name some common functional groups?

Certainly! Examples include hydroxyl (-OH), carboxyl (-COOH), and amino (-NH₂) groups. Each imparts unique properties to the compound.

So, are there families of compounds based on these groups?

Yes! Series of compounds sharing the same functional group are called homologous series. They show gradual changes in physical properties.

In summary, functional groups are claves in determining the behavior and properties of organic compounds.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Focusing on the unique tetravalency and catenation properties of carbon, this section highlights how these characteristics lead to a vast array of carbon-based compounds, known as organic compounds. The formation of saturated and unsaturated compounds is emphasized, demonstrating carbon's ability to bond with multiple elements.
Detailed
Organic Compounds
This section discusses the immense significance of organic compounds derived from carbon, emphasizing how their unique properties let them form a wide range of structures crucial to both life and daily use. Carbon, with its tetravalent nature, can bond with other carbon atoms leading to catenation, forming long chains, branched structures, or rings. This versatility results in a diverse array of organic compounds, including:
- Saturated Compounds: Carbon atoms are connected only by single bonds.
- Unsaturated Compounds: Carbon atoms that include double or triple bonds.
The ability of carbon to create stable molecules is underscored, showcasing its foundational role in biology, chemistry, and biochemistry. By explaining the unique bonding characteristics of carbon, this section lays the groundwork for understanding organic chemistry and its relevance.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Organic Compounds
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The two characteristic features seen in carbon, that is, tetravalency and catenation, put together give rise to a large number of compounds. Many have the same non-carbon atom or group of atoms attached to different carbon chains. These compounds were initially extracted from natural substances and it was thought that these carbon compounds or organic compounds could only be formed within a living system. That is, it was postulated that a ‘vital force’ was necessary for their synthesis. Friedrich Wöhler disproved this in 1828 by preparing urea from ammonium cyanate. But carbon compounds, except for carbides, oxides of carbon, carbonate and hydrogencarbonate salts continue to be studied under organic chemistry.
Detailed Explanation
Organic compounds are primarily carbon-based compounds that form due to carbon's unique properties. The two key features of carbon that allow for the vast array of organic compounds are:
- Tetravalency: This means that a carbon atom can form four bonds with other atoms, which allows it to connect with various elements, creating diverse structures with different chemical properties.
- Catenation: Carbon can form long chains by bonding to other carbon atoms. This enables the formation of straight, branched, or even ring-structured compounds.
Initially, it was believed that organic compounds could only be created in living organisms, a belief that was overturned by chemist Friedrich Wöhler's synthesis of urea from inorganic materials, proving that organic compounds could be formed in a laboratory setting too.
Examples & Analogies
Consider carbon like a versatile Lego block that can connect with different shapes and sizes (other atoms) to create complex structures. Just as you can build various models with a limited number of Lego pieces, the myriad combinations of carbon and other elements yield a vast array of organic compounds. This adaptability is what makes carbon the backbone of life on Earth.
Types of Carbon Compounds
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We have already seen the structure of methane. Another compound formed between carbon and hydrogen is ethane with a formula of C2H6. In order to arrive at the structure of simple carbon compounds, the first step is to link the carbon atoms together with a single bond and then use the hydrogen atoms to satisfy the remaining valencies of carbon. For example, the structure of ethane is arrived in the following steps:
- Step 1: C — C (Connecting carbon atoms with a single bond)
- Step 2: Each carbon atom remains unsatisfied in their valency, so each is bonded to three hydrogen atoms.
The electron dot structure of ethane is shown in a figure.
Detailed Explanation
Every organic molecule consists of carbon and hydrogen atoms among other elements. Let's break down the example of ethane (C2H6) into steps:
- Connecting Carbon Atoms: In the first step, we link two carbon atoms with a single bond, known as a covalent bond, which allows them to share their electrons.
- Fulfilling Valency with Hydrogen Atoms: After linking the carbon atoms, we need to attach hydrogen atoms to fill the remaining valencies. Since each carbon has four valencies, when two carbon atoms bond, we can attach three hydrogen atoms to each carbon to complete the structure, which gives us C2H6.
This method is fundamental in organic chemistry to understand how various carbon compounds are formed and their structures.
Examples & Analogies
Think of constructing a bridge with two supports (the carbon atoms) and connecting strings (hydrogen atoms) that hold them together to ensure stability. Just like a bridge needs to have its supports firmly connected and stable at both ends, carbon compounds need to have their valencies satisfied to maintain stability.
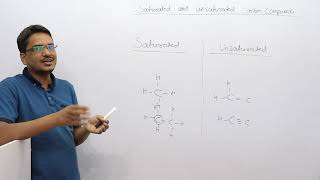
Saturated vs. Unsaturated Carbon Compounds
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
However, another compound of carbon and hydrogen has the formula C2H4 and is called ethene. We follow the same step-wise approach as above. Carbon-carbon atoms linked together with a single bond (Step 1). We see that one valency per carbon atom remains unsatisfied (Step 2). This can be satisfied only if there is a double bond between the two carbons (Step 3). Thus, compounds of carbon having double or triple bonds between the carbon atoms are known as unsaturated carbon compounds and they are more reactive than the saturated carbon compounds.
Detailed Explanation
In organic chemistry, compounds are categorized as saturated or unsaturated:
- Saturated Compounds: These compounds only contain single bonds between carbon atoms. For example, ethane (C2H6) is a saturated compound because it has single bonds only, which makes it less reactive.
- Unsaturated Compounds: These contain one or more double or triple bonds. For instance, ethene (C2H4) has a double bond between the two carbon atoms. This double bond makes unsaturated compounds more reactive than their saturated counterparts because of the additional energy and reactivity at the double bond site.
Examples & Analogies
Imagine saturated compounds like fully packed jars of jellybeans, where no more candy can fit in without removing some. Unsaturated compounds are like jars that have room for more jellybeans, as some spaces are available to add more candies (bonds). Therefore, if you want to mix in new flavors (reactants), it's easier to do so in the unsaturated jars.
Key Concepts
-
Catenation: Carbon's ability to form chains and rings through self-bonding.
-
Tetravalency: Carbon's capacity to make four covalent bonds, leading to diverse compounds.
-
Saturated Compounds: Molecules with only single carbon-carbon bonds.
-
Unsaturated Compounds: Molecules with one or more double or triple carbon-carbon bonds.
-
Functional Groups: Specific groups that alter the properties of organic compounds.
Examples & Applications
Methane (CH₄) is a simple saturated hydrocarbon.
Ethene (C₂H₄) is an unsaturated hydrocarbon with a double bond.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Carbon's tetravalent, it likes to bond, long chains and rings, it's never so fond!
Stories
Once upon a time, in the land of Chemistry, lived a carbon atom named Catie. She was known for her four friends - hydrogen, oxygen, nitrogen, and sulfur. Together they formed a group called organic compounds, making life possible everywhere!
Memory Tools
To remember functional groups, think 'C H A K' (Carboxyl, Hydroxyl, Amino, Ketone).
Acronyms
Remember 'SHU' for Properties
Saturated
Hydrocarbon
Unsaturated.
Flash Cards
Glossary
- Catenation
The ability of an atom to form bonds with itself, leading to chains and rings.
- Tetravalency
The property of carbon to form four covalent bonds with other atoms.
- Saturated Compounds
Compounds containing only single bonds between carbon atoms.
- Unsaturated Compounds
Compounds that contain one or more double or triple bonds between carbon atoms.
- Functional Groups
Specific groups of atoms that determine the chemical properties of compounds.
Reference links
Supplementary resources to enhance your learning experience.
