Versatile Nature of Carbon
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Carbon's Versatility
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are diving into why carbon is such an essential element in chemistry. Can anyone tell me what makes carbon unique compared to other elements?

I think it has to do with how it can bond with itself and other elements.

Exactly! This unique ability is known as **catenation**. Because of this, carbon can form long chains, branched structures, and even rings. This property allows carbon to create a multitude of compounds.

Wait, does it also have something to do with the number of bonds it can form?

That's right! Carbon is **tetravalent**, meaning it can form four strong bonds. This stability is essential for creating a wide variety of organic compounds. Can someone give me an example of a compound formed from carbon?

Methane! It’s one of the simplest carbon compounds.

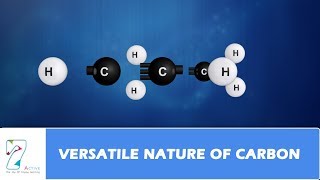
Great example! Methane (CH₄) is composed of one carbon atom bonded to four hydrogen atoms, illustrating carbon's tetravalency. Let's summarize: carbon's versatility comes from its ability to catenate and its tetravalency.
Understanding Saturated and Unsaturated Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss saturated and unsaturated compounds. What do you think distinguishes these two types?

I believe it has something to do with how many bonds there are between carbon atoms?

You're absolutely right! Saturated compounds have only single bonds connecting the carbon atoms, while unsaturated compounds have one or more double or triple bonds. This affects their stability and reactivity.

So, are saturated compounds less reactive?

Yes, saturated compounds tend to be less reactive than unsaturated ones. For instance, alkanes are saturated hydrocarbons, while alkenes and alkynes are unsaturated. Can anyone think of an example of an unsaturated compound?

Ethylene! It has a double bond between the carbon atoms.

Perfect! Ethylene is C₂H₄, illustrating the concept of unsaturation. So, to recap: saturated compounds have only single bonds and tend to be more stable, while unsaturated compounds include double or triple bonds and are more reactive.
Functional Groups and Their Role in Carbon Compounds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's explore functional groups in carbon compounds. Who can define what a functional group is?

I think a functional group is a group of atoms in a molecule that determines its chemical behavior.

Exactly! Functional groups influence the properties and reactions of compounds. For instance, adding an -OH group forms alcohols, which have different properties than hydrocarbons without that group.

So, if we have the same backbone of carbon atoms but different functional groups, they could have entirely different properties?

Correct! This leads to the concept of homologous series, where compounds share similar structural characteristics but differ in functional groups. Let's summarize: functional groups dictate the characteristics of carbon compounds and can change their reactivity.
Illustrating Carbon's Unique Characteristics
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let's look at how carbon's unique characteristics impact real-world applications. What are some everyday products that contain carbon?

Everything! From fuels to plastics!

Absolutely! Carbon forms the basis of fuels, plastics, and biological molecules that are crucial for life. This versatility profoundly influences the materials and compounds we use in daily life.

And we learned how carbon's ability to form various structures is essential for life's complexity!

Exactly! So, to recap, carbon's unique properties of catenation and tetravalency result in a vast array of compounds that are foundational to both organic chemistry and living organisms.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Carbon’s versatility is illustrated through its ability to form stable covalent bonds, both with itself and other elements, leading to a vast diversity of carbon compounds. Important features such as catenation and tetravalency enable carbon to form extensive chains and a variety of structures.
Detailed
Versatile Nature of Carbon
Carbon stands out as a unique element due to its remarkable ability to form numerous compounds; estimates suggest that chemists know millions of carbon compounds. This overwhelming abundance is largely attributed to two fundamental properties of carbon:
- Catenation: Carbon can form stable bonds with other carbon atoms to create long chains, branched chains, or ring structures. This capability, which is not exhibited by other elements to the same extent, allows for complex molecular architectures.
- Tetravalency: With four valence electrons, carbon can form strong, stable bonds with up to four other atoms, which may include other carbon atoms or various elements like hydrogen, oxygen, and nitrogen. This tetravalency contributes to the stability of carbon compounds and enhances their diversity.
The section elaborates on saturated and unsaturated compounds based on whether carbon atoms are singly bonded or include double or triple bonds. These structural differences affect their chemical properties and reactivity.
Furthermore, the presentation of functional groups and their roles in defining the characteristics of organic compounds emphasizes carbon's significance in forming complex structures vital for life. This section forms the foundation for understanding the molecular basis of organic chemistry.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Carbon Compounds
Chapter 1 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
We have seen the formation of covalent bonds by the sharing of electrons in various elements and compounds. We have also seen the structure of a simple carbon compound, methane.
Detailed Explanation
Carbon compounds are formed through covalent bonds, where atoms share their electrons. Methane (CH₄) is a simple example of a carbon compound where carbon shares its electrons with four hydrogen atoms. This sharing allows carbon to achieve a full outer shell, giving rise to stable molecules.
Examples & Analogies
Think of covalent bonding like a group of friends each holding hands. Each friend (atom) is secure in their position because they are all connected, just like the shared electrons secure the atoms in a molecule.
The Abundance of Carbon Compounds
Chapter 2 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In fact, we ourselves are made up of carbon compounds. The numbers of carbon compounds whose formulae are known to chemists was recently estimated to be in millions! This outnumbers by a large margin the compounds formed by all the other elements put together.
Detailed Explanation
Carbon is essential for life and forms the basis of many compounds in living organisms. The vast number of carbon compounds, estimated in millions, is due to carbon's unique properties. It can bond with itself (catenation) and with many other elements, leading to diverse molecular structures.
Examples & Analogies
Imagine a LEGO set. Each block is like a carbon atom that can connect with other blocks in countless ways, building everything from simple structures to complex designs, representing the variety of carbon compounds.
Catenation Property of Carbon
Chapter 3 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation.
Detailed Explanation
Catenation refers to carbon's ability to link to itself to form chains or rings. These structures can be long or complex and are the backbone of many organic molecules. This attribute makes carbon exceptionally versatile compared to other elements, enabling the formation of hydrocarbons and other compounds.
Examples & Analogies
Think of catenation like a chain made of paperclips. Each paperclip represents a carbon atom, and by linking them together, you can create a long chain. This chain can twist and turn, just like the various structures carbon can form.
Valency of Carbon
Chapter 4 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element.
Detailed Explanation
The valency of an element is the number of electrons it can share or bond with. Carbon's tetravalent nature means it can form four covalent bonds, allowing it to connect with multiple hydrogen atoms or other elements, resulting in a variety of compounds with differing properties.
Examples & Analogies
Imagine carbon as a table with four chairs around it. It can host four guests (other atoms), and depending on who sits down (the specific atoms chosen), the gathering could take on different characteristics or themes (properties of the resulting compound).
Types of Carbon Compounds
Chapter 5 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Compounds of carbon which are linked by only single bonds between the carbon atoms are called saturated compounds. Compounds of carbon having double or triple bonds between their carbon atoms are called unsaturated compounds.
Detailed Explanation
Saturated compounds have only single bonds between carbon atoms, making them stable and less reactive. In contrast, unsaturated compounds contain double or triple bonds, which add reactivity to the molecules. This difference impacts their chemical behavior in reactions.
Examples & Analogies
Think of saturated compounds like a garden where plants are neatly arranged (stable and organized), while unsaturated compounds are like a wild jungle (more chaos and reaction potential) with twists and turns in the way the plants are arranged.
Importance of Carbon Bonding
Chapter 6 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The bonds that carbon forms with most other elements are very strong making these compounds exceptionally stable.
Detailed Explanation
Carbon's small atomic size allows it to form strong covalent bonds with other elements. This stability is crucial for the formation of complex molecules essential for life, enabling interactions and reactions necessary for biological processes.
Examples & Analogies
Think of carbon-carbon bonds like strong steel cables supporting a bridge. This strength allows the bridge (carbon compounds) to support heavy loads (biological functions), ensuring its stability and reliability in various conditions.
Organic Compounds and Their Origin
Chapter 7 of 7
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Many have the same non-carbon atom or group of atoms attached to different carbon chains. These compounds were initially extracted from natural substances and it was thought that these carbon compounds or organic compounds could only be formed within a living system.
Detailed Explanation
Organic compounds are characterized by the presence of carbon along with other elements. Historically, it was believed that these compounds could only arise from living organisms, but this notion was disproven when synthetic methods created organic molecules in labs.
Examples & Analogies
This is akin to initially believing that only cakes baked in an oven could taste sweet. Once people started experimenting with different cooking techniques, they discovered that sweet flavors could be created in many other ways beyond baking.
Key Concepts
-
Catenation: Carbon's ability to bond with itself to form extensive structures.
-
Tetravalency: Carbon's capability to form four bonds with other atoms.
-
Saturated Compounds: Compounds with single bonds only.
-
Unsaturated Compounds: Compounds with double or triple bonds between carbon atoms.
-
Functional Groups: Groups that define the chemical behavior of organic compounds.
-
Homologous Series: A series of compounds that have the same functional group but differ in carbon chain length.
Examples & Applications
Methane (CH₄) as an example of a saturated compound.
Ethylene (C₂H₄) illustrating the concept of an unsaturated compound with a double bond.
Ethanol (C₂H₅OH) demonstrating the functional group of alcohol.
Butanoic acid showing the carboxylic acid functional group.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Carbon's special tricks, with chains it mixes; four bonds to hold, making life unfold.
Stories
Imagine carbon as a talented architect, building vast structures with its ability to bond with itself, creating everything from long chains to intricate rings.
Memory Tools
For remembering saturated vs. unsaturated: 'Single Bonds are Safe, Double Bonds are Dangerous.'
Acronyms
CARBON
Catenation and Reactivity; Bonds of all types form Organic Nurturing.
Flash Cards
Glossary
- Catenation
The ability of carbon atoms to bond with one another to form long chains, branches, or rings.
- Tetravalency
The property of carbon where it can form four covalent bonds due to having four valence electrons.
- Saturated Compounds
Carbon compounds that contain only single bonds between carbon atoms.
- Unsaturated Compounds
Carbon compounds that contain one or more double or triple bonds between carbon atoms.
- Functional Groups
Specific groups of atoms that confer characteristic properties and reactivity to organic compounds.
- Homologous Series
A series of compounds that have the same functional group and similar chemical properties, differing only in the carbon chain length.
Reference links
Supplementary resources to enhance your learning experience.
