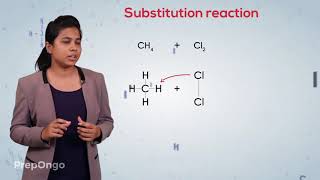
Substitution Reaction
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Substitution Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss substitution reactions, particularly in saturated hydrocarbons. Can anyone tell me what a substitution reaction is?

Is it when one atom in a molecule is replaced by another atom?

Exactly! In substitution reactions, one atom or group of atoms in a molecule is replaced by another. For instance, when chlorine reacts with alkanes under sunlight, what do you think happens?

The chlorine replaces the hydrogen atoms, right?

Yes! And this replacement occurs one hydrogen atom at a time. Let’s remember this with the mnemonic 'C-H Swap': Chlorine takes Hydrogen in a Substitution.

So, could you give me an example of this?

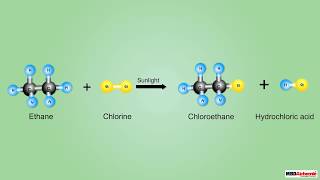
Certainly! Take ethane (C2H6) reacting with chlorine. The equation looks like this: C2H6 + Cl2 → C2H5Cl + HCl. Any questions?
Mechanism of Substitution Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's explore the mechanism of substitution reactions. Can anyone explain how the reaction proceeds?

Does it involve forming free radicals?

Correct! When chlorine gas is irradiated with sunlight, it dissociates into free radicals. These radicals are highly reactive and will attack the ethane molecule.

So, what happens at each step?

Initially, one H atom is replaced by Cl, producing chloroethane and HCl. This process can continue, leading to multiple chlorinated products. Remember, this is a chain reaction!

Can we represent this chain process?

Sure! The overall reaction is free-radical substitution. Think of it as a relay race, where one runner hands the baton, and the next takes over!
Examples of Substitution Reactions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Examples are crucial for understanding. Who can recall a few reactions of alkanes with halogens?

I know methane releases HCl when it reacts with chlorine!

Excellent! The reaction is CH4 + Cl2 → CH3Cl + HCl. Methane is highly abundant; therefore, substitution reactions are significant in organic synthesis.

Are there any safety concerns with these reactions?

Good question! Chlorine gas is toxic, and reactions should be contained due to the release of HCl. Always follow safety protocols in lab environments.

So, in terms of practical applications, how are these reactions useful?

Substitution reactions are integral in producing industrial chemicals, like solvents and pesticides. Thus, they're key in many applications!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Saturated hydrocarbons typically display low reactivity, but under specific conditions, such as exposure to sunlight, they can undergo substitution reactions where chlorine replaces hydrogen atoms. This leads to the formation of various chlorinated hydrocarbons alongside hydrochloric acid as a byproduct.
Detailed
In this section, we delve into substitution reactions, particularly focusing on saturated hydrocarbons such as alkanes. These compounds are usually inert to various reagents but show reactivity when exposed to chlorine in the presence of sunlight. The chlorine replaces hydrogen atoms in the hydrocarbon molecules sequentially, leading to the formation of chlorinated derivatives. For example, the reaction of ethane (C2H6) with chlorine results in the substitution of hydrogen with chlorine, forming chloroethane (C2H5Cl) and hydrochloric acid (HCl) as a byproduct. This mechanism is significant as it showcases an essential aspect of organic chemistry, where the stability of saturated hydrocarbons is altered through such reactions.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Substitution Reactions
Chapter 1 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Saturated hydrocarbons are fairly unreactive and are inert in the presence of most reagents. However, in the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction.
Detailed Explanation
Saturated hydrocarbons, like alkanes, do not easily react with most chemicals because their bonds are strong and stable. However, when exposed to sunlight, they can react with chlorine gas. In this reaction, chlorine atoms can replace hydrogen atoms in the hydrocarbon, leading to the formation of new compounds. This type of reaction is called a substitution reaction because one type of atom or group of atoms takes the place of another in a molecule.
Examples & Analogies
Think of this reaction like replacing the batteries in a flashlight. When you take out old batteries (hydrogen) and put in new, charged batteries (chlorine), the flashlight (hydrocarbon compound) still works but now has a different source of energy.
Example of a Substitution Reaction
Chapter 2 of 2
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Chlorine can replace the hydrogen atoms one by one. It is called a substitution reaction because one type of atom or a group of atoms takes the place of another. A number of products are usually formed with the higher homologues of alkanes.
CH₄ + Cl₂ → CH₃Cl + HCl (in the presence of sunlight)
Detailed Explanation
This chunk describes a specific example of a substitution reaction involving methane (CH₄), the simplest alkane. When methane reacts with chlorine (Cl₂) in the presence of sunlight, one hydrogen atom from methane is replaced by a chlorine atom. This results in the formation of chloromethane (CH₃Cl), and hydrochloric acid (HCl) is released as a byproduct. This process may continue, replacing more hydrogen atoms with chlorine, leading to multiple products through further substitution.
Examples & Analogies
Imagine you have a fruit salad (methane) where you can replace apples (hydrogens) with oranges (chlorines). Every time you swap an apple for an orange, your fruit salad looks different, and if you keep swapping, you can end up with all oranges and no apples. Similarly, in substitution reactions, continuing the process can lead to various products.
Key Concepts
-
Substitution Reactions: Involves the replacement of atoms in molecules.
-
Saturated Hydrocarbons: Compounds with single bonds; generally more stable and less reactive.
-
Chlorination: A specific type of substitution where chlorine is added to alkanes.
Examples & Applications
The substitution of methane (CH4) with chlorine results in chloroform (CHCl3) and hydrochloric acid (HCl).
C2H6 + Cl2 → C2H5Cl + HCl illustrates the substitution reaction in ethane.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Chlorine comes to play, taking hydrogen away, in sunlight's bright array.
Stories
Once upon a time, in a glowing garden, a mischievous chlorine played tag with hydrogen, replacing it one by one until it became chloroethane.
Memory Tools
C-H Swap: Chlorine Takes Hydrogen.
Acronyms
S-R-C
Substitution-Reaction-Chlorination.
Flash Cards
Glossary
- Substitution Reaction
A reaction where one atom in a molecule is replaced by another atom or group of atoms.
- Saturated Hydrocarbon
A hydrocarbon that contains only single bonds between carbon atoms.
- Chlorination
A specific type of substitution reaction where chlorine replaces hydrogen in hydrocarbons.
Reference links
Supplementary resources to enhance your learning experience.
