Dalton’s Atomic Theory
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Indivisibility of Atoms
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today we're going to explore Dalton's Atomic Theory. To start, does anyone know what we mean when we say 'matter is made of atoms'?

Isn't that like saying everything is made of tiny particles?

Exactly! Dalton proposed that these particles, or what he called atoms, are indivisible. This means they cannot be split into smaller parts. We often remember this by the acronym I.A.M. — Indivisible Atoms Matter.

So, atoms are the smallest units of matter?

Correct! And according to Dalton, all atoms of a particular element are identical. Can you think of any examples?

All oxygen atoms are identical, right?

Yes! They're the same in mass and properties. Let's remember that with the mnemonic: 'All Atoms Are Alike' or A.A.A.!

What about atoms of different elements?

Great question! Atoms of different elements have different masses and properties. This is fundamental to understanding chemical reactions and combining substances.

To recap, Dalton's concept of indivisible atoms forms the foundation of chemistry as we know it. Can anyone summarize what we’ve learned so far?

Matter is made up of indivisible atoms that are identical for each element!
Compounds and Fixed Ratios
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's move onto the second key point of Dalton's theory: compounds are formed when atoms of different elements combine in fixed ratios. Does anyone understand what that means?

It means there are specific ways atoms can combine, right?

Exactly! Dalton stated that when elements combine to form compounds, they do so in fixed proportions by mass, known as the Law of Definite Proportions. We often use RFM here — Regularly Fixed Masses.

Can you give us an example?

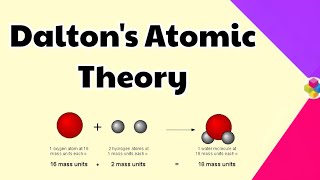
Sure! Water is a classic example. It always consists of two hydrogen atoms and one oxygen atom, or H2O. That's a fixed ratio!

So if I have too much hydrogen or oxygen, I wouldn't get water?

Correct! Excess or deficiency in any element would prevent proper compound formation. This leads us to remember: 'Proportions Matter'.

To summarize, Dalton emphasized that compounds are always formed in fixed ratios which are consistent. Can anyone reiterate our key point?

Compounds are formed from elements in fixed proportions, like H2O from 2 hydrogen and 1 oxygen!
Chemical Reactions and Atom Conservation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about the final postulate: chemical reactions reorganize atoms. What does everyone think this means?

Does it mean atoms are rearranged during reactions?

Exactly! Atoms are neither created nor destroyed during a reaction; they are simply rearranged. This adheres to the Law of Conservation of Mass. A good way to remember this is: C.A.D. — Conservation Atoms Dissipate.

What does 'law of conservation of mass' mean in this context?

It means the mass of the products in a chemical reaction must equal the mass of the reactants. If 5g of hydrogen reacts with 8g of oxygen, you'll have 13g of water!

So even if we can't see the atoms, we know they follow these rules?

Precisely! To wrap things up, who can summarize our discussion about atom conservation in chemical reactions?

Atoms are rearranged in reactions, but not created or destroyed, maintaining total mass!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
John Dalton's Atomic Theory, published in 1808, posited that all matter is made of small indivisible particles called atoms, which are identical for each element and differ among elements in mass. This theory provided a framework that could explain the laws of chemical combination, although it initially fell short of explaining certain phenomena related to gases.
Detailed
Dalton’s Atomic Theory
In 1808, John Dalton presented his groundbreaking work titled "A New System of Chemical Philosophy" which articulated the concept that all matter is composed of indivisible atoms. Dalton's theory is built on four main postulates:
1. Indivisibility of Atoms: Matter consists of indivisible atoms, meaning that atoms cannot be created or destroyed in chemical reactions.
2. Identical Properties: All atoms of a given element have identical properties, including mass. Atoms of different elements differ in mass.
3. Formation of Compounds: Compounds are formed when atoms of different elements combine in fixed ratios, which aligns with the Law of Definite Proportions.
4. Reorganization in Reactions: Chemical reactions involve the reorganization of atoms; atoms are neither created nor destroyed, supporting the Law of Conservation of Mass.
Despite its strengths in explaining foundational chemical laws, Dalton's theory could not account for specific behaviors observed in gases or elucidate the reasons behind the combining of atoms, leading to the development of more comprehensive theories in later years.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Dalton’s Atomic Theory
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Although the origin of the idea that matter is composed of small indivisible particles called ‘a-tomio’ (meaning, indivisible), dates back to the time of Democritus, a Greek Philosopher (460–370 BC), it again started emerging as a result of several experimental studies which led to the laws mentioned above.
Detailed Explanation
Dalton's Atomic Theory is rooted in ancient ideas, particularly those proposed by Democritus, who suggested that matter consists of tiny, indivisible particles. Dalton revived this idea in the early 19th century after extensive scientific developments, laying the groundwork for modern chemistry by emphasizing that atoms are the fundamental building blocks of matter.
Examples & Analogies
Imagine building a house. Just as bricks are the fundamental units that make up a house, atoms are like the bricks of all matter around us. If you want to understand how the house (matter) is structured, you must understand the properties and arrangements of the bricks (atoms).
Key Postulates of Dalton’s Atomic Theory
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In 1808, Dalton published ‘A New System of Chemical Philosophy’, in which he proposed the following:
1. Matter consists of indivisible atoms.
2. All atoms of a given element have identical properties, including identical mass. Atoms of different elements differ in mass.
3. Compounds are formed when atoms of different elements combine in a fixed ratio.
4. Chemical reactions involve reorganisation of atoms. These are neither created nor destroyed in a chemical reaction.
Detailed Explanation
Dalton's Atomic Theory introduced four essential concepts:
1. Indivisibility of Atoms: Atoms cannot be split into smaller parts by chemical means.
2. Identical Mass of Atoms: Every atom of a given element is the same, while atoms of different elements have different masses.
3. Fixed Ratios in Compounds: Atoms combine in specific ratios to form compounds, which reflects the consistency in chemical composition.
4. Conservation of Atoms in Reactions: During chemical reactions, atoms are rearranged, but none are created or destroyed; they simply form new compounds.
Examples & Analogies
Think of building different types of furniture from wooden blocks. Each type of furniture (like a chair or a table) must use specific arrangements of these wooden blocks (atoms) in set ratios. Just as you cannot create or erase blocks when making your furniture, in chemistry, atoms remain unchanged during reactions—it’s the arrangements that change.
Limitations of Dalton’s Atomic Theory
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Dalton’s theory could explain the laws of chemical combination; however, it could not explain the laws of gaseous volumes. It could not provide the reason for combining of atoms, which was answered later by other scientists.
Detailed Explanation
While Dalton's Atomic Theory laid a fundamental foundation and explained many aspects of chemical reactions and combinations, it had its limitations.
- It struggled to explain the behavior of gases, which did not always conform to the ratios in which they combined. For instance, Dalton could not account for why two volumes of hydrogen gas combined with one volume of oxygen gas to create water, despite the fact that the masses involved suggested otherwise.
- Future scientists built on these shortcomings to develop a more nuanced understanding of atomic behavior, integrating ideas about molecular structure and bonding.
Examples & Analogies
Consider trying to fit puzzle pieces into a picture. Dalton's theory was like understanding that each piece relates to the overall image, but it didn’t account for how some pieces might fit together differently depending on their shape or edges. Later scientists looked closer at the 'shapes' (or structures and bonds) of atoms to explain these fit variations better.
Key Concepts
-
Indivisible Atoms: Matter is composed of atoms that cannot be divided.
-
Identical Atoms: All atoms of an element are identical in mass and properties.
-
Compounds Formed by Fixed Ratios: Atoms combine in fixed proportions to form compounds.
-
Conservation of Atoms: Atoms are rearranged in chemical reactions but are not created or destroyed.
Examples & Applications
Water (H2O) is a compound formed from two hydrogen atoms and one oxygen atom, exemplifying fixed ratios.
During combustion, the rearrangement of molecules shows that the total mass from reactants equals the products, following the Law of Conservation of Mass.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Atoms are small, atoms are stout, they make up matter without a doubt!
Stories
Imagine a tiny world where each atom is a unique character, forming bonds with others to create compounds, a playful dance in chemistry!
Memory Tools
IAAC: Indivisible Atoms Are Clear - remember atoms are indivisible according to Dalton.
Acronyms
C.M.A. for Dalton's theory
Conservation of Mass and Atoms.
Flash Cards
Glossary
- Atom
The smallest unit of matter that retains the properties of an element.
- Compound
A substance formed when two or more elements combine in fixed proportions.
- Law of Definite Proportions
A law stating that a given compound always contains the same elements in the same proportions by mass.
- Law of Conservation of Mass
A principle stating that mass is neither created nor destroyed in a chemical reaction.
Reference links
Supplementary resources to enhance your learning experience.
