Uncertainty in Measurement
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Uncertainty in Measurement
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to tackle the idea of uncertainty in measurements. Can anyone tell me why uncertainty is important in science?

I think it's important because measurements can be wrong.

Exactly! Precision and reliability are critical in science. Uncertainty acknowledges that we can't measure anything perfectly.

What do we do to deal with that uncertainty?

We use significant figures! They help us express the precision of our measurements.

How do you know how many significant figures to use?

Great question! There are rules for determining them that we’ll explore next.

_Summary_: Uncertainty reflects the limitations of measuring devices, and significant figures help convey the precision of those measurements.
Scientific Notation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s tackle scientific notation. Why do chemists prefer it?

It makes big numbers easier to work with!

Correct! For example, instead of writing 602,000,000,000,000,000,000, we write 6.022 × 10^23.

And how do you convert them back?

To convert, just reverse the process. Move the decimal to make it complete based on the exponent!

Can you give an example using a small number?

Certainly! For 0.000000000000000000000166, we can express it as 1.66 × 10^-22. Remember that in scientific notation, N has to be between 1 and 10.

_Summary_: Scientific notation simplifies the handling of measurements that span a wide range of magnitudes.
Significant Figures
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s dive deeper into significant figures. Who can tell me how they determine the number of significant figures in a measurement?

I know that all non-zero digits are significant!

Exactly, and what about zeros?

Leading zeros aren’t significant, but zeros between non-zero digits are!

Correct! Don’t forget that trailing zeros count if there’s a decimal point. Can anyone provide an example?

In 100.0, there are four significant figures right?

Right! Meanwhile, in 100, there’s only one. _Summary_: Significant figures provide a way to express precision and reflect the certainty of measurements.
Dimensional Analysis
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Lastly, let’s discuss dimensional analysis. What can it help us accomplish?

It helps convert one unit to another without losing accuracy!

Exactly! If we know that 1 inch equals 2.54 cm, how would you use that to convert 3 inches to centimeters?

By multiplying 3 by 2.54 cm/inch.

Spot on! Unit factors make this process systematic and clear. _Summary_: Dimensional analysis ensures we can accurately convert units while maintaining precision.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Uncertainty in measurement plays a crucial role in chemistry as it affects the accuracy and precision of experimental data and theoretical calculations. This section delves into scientific notation, significant figures, and dimensional analysis, which are essential for effectively handling experimental values.
Detailed
In this section, we dissect the critical theme of uncertainty in measurement which arises from limitations of measuring instruments and human factors. Accurate measurements are foundational in chemistry for achieving reliable results and conclusions. To facilitate the handling of numbers that vary widely in scale, scientists utilize scientific notation, allowing large and small numbers to be expressed conveniently. The section also addresses significant figures, denoting meaningful digits that represent the precision of measurements. Such understanding is vital as it helps in accurately reporting experimental data and its potential uncertainties. Finally, we introduce dimensional analysis, a method of converting units systematically, further supporting the accuracy and clarity needed in scientific communication.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Scientific Notation
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As chemistry is the study of atoms and molecules, which have extremely low masses and are present in extremely large numbers, a chemist has to deal with numbers as large as 602,200,000,000,000,000,000 for the molecules of 2 g of hydrogen gas or as small as 0.00000000000000000000000166 g mass of a H atom. Similarly, other constants such as Planck’s constant, speed of light, charges on particles, etc., involve numbers of the above magnitude. It may look funny for a moment to write or count numbers involving so many zeros but it offers a real challenge to do simple mathematical operations of addition, subtraction, multiplication or division with such numbers. This problem is solved by using scientific notation for such numbers, i.e., exponential notation in which any number can be represented in the form N × 10ⁿ, where n is an exponent having positive or negative values and N is a number (called digit term) which varies between 1.000... and 9.999.... Thus, we can write 232.508 as 2.32508 × 10² in scientific notation. Note that while writing it, the decimal had to be moved to the left by two places and same is the exponent (2) of 10 in the scientific notation. Similarly, 0.00016 can be written as 1.6 × 10⁻⁴. Here, the decimal has to be moved four places to the right and (–4) is the exponent in the scientific notation.
Detailed Explanation
The scientific notation is a method used to efficiently represent very large or very small numbers. In chemistry, we often deal with quantities that are far beyond the scope of ordinary numbers. For instance, the number of molecules in a simple substance like water can be extraordinarily large, requiring many zeros if expressed in standard decimal form. To simplify this, we can express these quantities in a more manageable way. Scientific notation allows us to express any number as a number between 1 and 10 (the digit term) multiplied by an exponent of 10 (the power term). Therefore, instead of writing a long number with many zeros, we can write it concisely and make calculations easier. For example, instead of saying there are '602,200,000,000,000,000,000' molecules of hydrogen in 2g, we write '6.022 × 10²⁴', which is much simpler.
Examples & Analogies
Think of scientific notation as a way to write addresses in a simplified manner. Just like we might use abbreviations for a long street name, scientific notation helps simplify very long numbers. If you lived on 'One Thousand Five Hundred and Sixty-Seven Long Distance Avenue', you could say you live at '1567 Long Distance Ave' instead. This gives an exact reference with much less clutter, making it easier to communicate.
Significant Figures
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
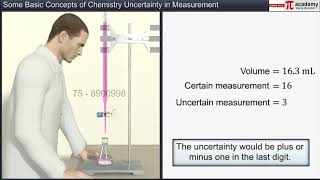
The uncertainty in the experimental or the calculated values is indicated by mentioning the number of significant figures. Significant figures are meaningful digits which are known with certainty plus one which is estimated or uncertain. The uncertainty is indicated by writing the certain digits and the last uncertain digit. Thus, if we write a result as 11.2 mL, we say the 11 is certain and 2 is uncertain and the uncertainty would be +1 in the last digit. Unless otherwise stated, an uncertainty of +1 in the last digit is always understood. There are certain rules for determining the number of significant figures.
Detailed Explanation
Significant figures play a crucial role in scientific measurement as they reflect the precision of our measurements. The idea is to communicate how reliable our measurements are and how much confidence we have in them. When we perform calculations, the result should convey only as much certainty as the least precise measurement used in the calculation. For example, if we measure with a ruler that only goes to the millimeter, we cannot express a result with micrometer precision, because that would imply we know that much more than we can actually measure. Therefore, we follow certain rules that dictate which digits count towards the significant figures. These include counting non-zero digits, zeros between non-zero digits, and zeros following a decimal point after a non-zero digit as significant, while excluding leading zeros.
Examples & Analogies
Imagine you are cooking and using a measuring cup. If you say you used 'one cup of sugar', that is a precise measurement. If you try to estimate using 'a bit of sugar', that doesn't tell us how much you really used. Similarly, in measurements, certainty comes from counting significant figures, helping to indicate how exact your measurement is, just like saying 'one whole cup' gives more information than 'some sugar'.
Dimensional Analysis
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Often while calculating, there is a need to convert units from one system to the other. The method used to accomplish this is called factor label method or unit factor method or dimensional analysis.
Detailed Explanation
Dimensional analysis is a powerful method in chemistry that allows us to convert one unit of measurement into another using conversion factors. This process involves recognizing that different units can be used to describe the same quantity. For example, if you want to convert inches into centimeters, you can use the conversion factor that 1 inch equals 2.54 centimeters. By multiplying your measurement in inches by this conversion factor, you can arrive at the equivalent measurement in centimeters. It's essential for ensuring accuracy when making measurements and calculations because using the correct unit is crucial in scientific work.
Examples & Analogies
You can think of dimensional analysis as following a recipe in cooking. When cooking, if a recipe calls for ingredients in cups and you only have a measuring spoon, you need to convert those measurements. Just like 1 cup = 16 tablespoons, in dimensional analysis, we switch units appropriately to achieve the desired quantity. It ensures we get our recipe right, just as it helps scientists communicate quantities accurately.
Key Concepts
-
Uncertainty: Reflects imprecision in measurements and is important for scientific accuracy.
-
Scientific Notation: A shorthand representation that makes working with very large or small numbers manageable.
-
Significant Figures: Indicate the precision of a measurement and maintain clarity in reporting the results.
-
Dimensional Analysis: A technique for converting units systematically while preserving accuracy.
Examples & Applications
Example of scientific notation: Writing 602000000000000000000 as 6.022 × 10^23.
Example of significant figures: In the measurement 0.00520, there are three significant figures.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Measure twice, report it right, significant figures are by our sight.
Stories
Once upon a time, a chemist found a tiny atom, so they counted its weight but needed to write it right. Thus, they wrote it in scientific form, making it easy to transform.
Memory Tools
SIMPLe: Scientific In Measurement Precision and Length for socks, shoes, and more!
Acronyms
SI
Significant Importance in measurements!
Flash Cards
Glossary
- Uncertainty
The doubt that exists about the result of any measurement.
- Scientific Notation
A method of expressing numbers as a product of a coefficient and a power of ten.
- Significant Figures
Digits in a number that are important for conveying the precision of a measurement.
- Dimensional Analysis
A mathematical technique used to convert from one unit of measurement to another.
Reference links
Supplementary resources to enhance your learning experience.
