Empirical Formula for Molecular Formula
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Empirical and Molecular Formulas
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to dive into the concepts of empirical and molecular formulas. Can anyone tell me what an empirical formula represents?

It represents the simplest ratio of elements in a compound, right?

Exactly! For instance, if a compound has the formula C2H4, what would its empirical formula be?

It would be CH because that's the simplest ratio.

Great! Now, who can tell me what a molecular formula indicates?

It shows the actual number of atoms of each element in a molecule.

Correct! So, C2H4 is a molecular formula indicating two carbon and four hydrogen atoms. Remember, the empirical formula gives us a ratio, while the molecular formula gives specific counts.

What if I want to determine the molecular formula of a compound, then?

To find that, you first determine the empirical formula and then compare its mass with the compound's molar mass. Let’s see how we can do that!

To recap: An empirical formula is the simplest ratio while the molecular formula shows actual atoms. Now, let's practice determining these formulas from given compositions.
Calculating Empirical Formulas
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s apply our knowledge to practical scenarios. Suppose a compound is composed of 4.07% hydrogen, 24.27% carbon, and 71.65% chlorine. How do we start?

We can assume we have 100 grams of the compound to make calculations easier.

Exactly! That means we have 4.07 g of hydrogen, 24.27 g of carbon, and 71.65 g of chlorine. What’s the next step?

Next, we divide each mass by the respective atomic masses!

Right again! What are the atomic masses we use?

Hydrogen is about 1.008 g/mol, carbon is about 12.01 g/mol, and chlorine is about 35.45 g/mol.

Perfect! Now, calculate the moles for each element.

For hydrogen, that's 4.07 g divided by 1.008 g/mol, which gives us approximately 4.04 moles.

Great work. Continue with the others?

Sure! For carbon: 24.27 g divided by 12.01 g/mol equals about 2.02 moles. For chlorine: 71.65 g divided by 35.45 g/mol gives around 2.02 moles too.

Exactly! Now, what’s next?

We find the mole ratio by dividing each by the smallest number, which is 2.02.

Fantastic! What are the ratios then?

The ratio ends up being about 2 for hydrogen and 1 for both carbon and chlorine.

So the empirical formula would be CH2Cl. Don’t forget to keep this process in mind, as it’s crucial in understanding compound structures!
Determining the Molecular Formula
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we have our empirical formula, let’s move on to determining the molecular formula. If the molar mass of this compound is given as 98.96 g/mol, what's our first step?

First, calculate the empirical formula mass for CH2Cl.

Correct! Can anyone help with that calculation?

The empirical formula mass would be 12.01 g/mol for carbon plus 2 times 1.008 g/mol for hydrogen plus 35.45 g/mol for chlorine.

Let’s calculate that.

That totals to about 49.48 g/mol.

Now compare this with the given molar mass of 98.96 g/mol. What's the next step?

We divide the molar mass by the empirical formula mass. That’s 98.96 g/mol divided by 49.48 g/mol, which gives us 2.

Perfect! This number tells us how many times the empirical formula fits into the molecular formula. So what’s the molecular formula?

It's C2H4Cl2!

Exactly! Remember, finding both the empirical and molecular formulas is essential in chemistry as it provides insights into the composition and structure of compounds. Well done today!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In this section, we learn that an empirical formula represents the simplest whole number ratio of different atoms in a compound, while the molecular formula indicates the exact number of each type of atom. We also explore the methods to derive these formulas from mass percent compositions and the compound's molar mass.
Detailed
Overview
The empirical formula is the simplest representation of the ratio of elements in a compound, while the molecular formula conveys the actual number of atoms of each element in a molecule. Understanding the difference is crucial when analyzing chemical compounds.
Key Points
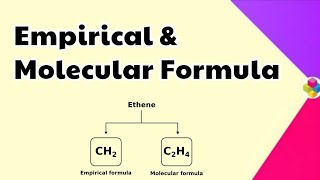
- Empirical Formula: Represents the simplest whole number ratio of atoms in a compound (e.g., CH for ethylene C2H4).
- Molecular Formula: Shows the exact number of atoms of each element in a molecule (e.g., C2H4).
- Determining Formulas: To find the empirical formula, mass percent composition is converted to moles, and then the mole ratio is derived. For the molecular formula, the empirical formula mass is compared to the given molar mass of the compound.
Example Illustration
For a compound with 4.07% hydrogen, 24.27% carbon, and 71.65% chlorine, the steps to derive its empirical and molecular formulas include:
- Convert mass per cent to grams by assuming 100 g of the compound,
- Calculate moles for each element,
- Divide by the smallest mole value to form a ratio,
- Use the molar mass to find the molecular formula by determining how many times the empirical formula mass fits into the molar mass.
Overall, this section emphasizes the calculation techniques necessary for determining empirical and molecular formulas it also highlights their significant roles in chemistry, particularly in molecular compound characterization.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Empirical and Molecular Formulas
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An empirical formula represents the simplest whole number ratio of various atoms present in a compound, whereas, the molecular formula shows the exact number of different types of atoms present in a molecule of a compound.
Detailed Explanation
The empirical formula gives us the simplest form of the ratio of atoms in a compound. For example, in glucose (C6H12O6), the empirical formula is CH2O, which indicates that for every carbon atom, there are two hydrogen atoms and one oxygen atom. The molecular formula, however, tells us the actual number of atoms in a molecule, which in the example of glucose is six carbons, twelve hydrogens, and six oxygens.
Examples & Analogies
Think of a recipe for a cake. The empirical formula is like saying you need 1 cup of flour, 2 eggs, and 1 cup of sugar to make the cake (to get the simplest version). The molecular formula gives the exact amounts required for a specific cake, such as 3 cups of flour, 6 eggs, and 3 cups of sugar to serve a large gathering.
Determining Empirical Formula from Mass Percentages
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If the mass per cent of various elements present in a compound is known, its empirical formula can be determined.
Detailed Explanation
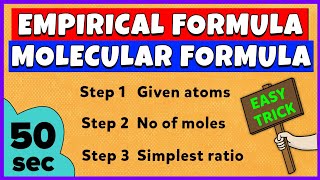
To find the empirical formula from mass percentages, you first convert those percentages into grams (assuming 100 g of the compound). Then, divide the mass of each element by its atomic mass to get the number of moles. Once you have the number of moles for each element, divide each by the smallest number of moles to obtain the simplest ratio. Finally, use those ratios to write the empirical formula.
Examples & Analogies
Imagine you are trying to create a new smoothie. If you have 40 g of strawberries, 20 g of bananas, and 40 g of yogurt, you would first determine how many 'parts' you have based on the smallest ingredient. Here, the banana is lowest (20 g), so you have a 2:1:2 ratio of strawberries to bananas to yogurt. Therefore, the smoothie recipe would be 2 parts strawberries, 1 part banana, and 2 parts yogurt.
Finding Molecular Formula from Empirical Formula
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Molecular formula can further be obtained if the molar mass is known.
Detailed Explanation
Once the empirical formula is established, the next step is to calculate its empirical formula mass by adding up the atomic masses of the atoms in the empirical formula. Then, divide the compound's molar mass (the mass of one mole of the compound) by the empirical formula mass to find a whole number 'n'. Multiply the subscripts in the empirical formula by this number to get the molecular formula.
Examples & Analogies
Imagine that after determining the smoothie recipe for a regular serving (one cup), you find that to serve a whole batch (12 cups), you need 12 times the amount of each ingredient. If your empirical recipe says 2 strawberries, 1 banana, 2 yogurts, by multiplying 2 by 12, 1 by 12, and 2 by 12, you find you'll need 24 strawberries, 12 bananas, and 24 yogurts for your party.
Example Problem: Finding Empirical and Molecular Formulas
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A compound contains 4.07% hydrogen, 24.27% carbon and 71.65% chlorine. Its molar mass is 98.96 g. What are its empirical and molecular formulas?
Detailed Explanation
To solve the problem, we first treat the percentages as grams. We then calculate moles: for hydrogen, 4.07 g / 1.008 g/mol = 4.04 moles; for carbon, 24.27 g / 12.01 g/mol = 2.02 moles; for chlorine, 71.65 g / 35.453 g/mol = 2.02 moles. Dividing all by the smallest (2.02) gives us a ratio of 2:1:2. Thus, the empirical formula is C1H2Cl2. Next, we find the empirical formula mass (49.48 g), divide the molar mass (98.96 g) by the empirical formula mass to get n = 2, giving us C2H4Cl2 as the molecular formula.
Examples & Analogies
Consider making a fruit salad with a mix of fruits. The percentages you started with tell you how much fruit to use; when you mix it all together in larger quantities for a party, you're applying the same ratios on a larger scale, just like finding the molecular formula after establishing the empirical ratio.
Key Concepts
-
Empirical Formula: Simplest ratio of elements in a compound.
-
Molecular Formula: Exact number of atoms of each element in the compound.
-
Mass Percent Composition: Calculation basis for deriving empirical formulas.
-
Molar Mass: Needed to determine the molecular formula from the empirical formula.
Examples & Applications
The empirical formula of hydrogen peroxide (H2O2) is HO.
For a compound containing 40% carbon, 6.71% hydrogen, and 53.29% oxygen, the empirical formula can be derived through mass to moles conversion.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Empirical is simple, just a ratio you see, / Molecular gives exact counts, that's the key!
Stories
Imagine two chefs cooking: one keeps it simple with a tiny list (empirical) while the other lists every ingredient (molecular). Selection matters!
Memory Tools
For Empirical, think E for Easy (ratio); for Molecular, M for More (actual numbers).
Acronyms
Use 'E=M' to remember that Empirical is a ratio while Molecular is the exact count that matters.
Flash Cards
Glossary
- Empirical Formula
The simplest whole number ratio of the different types of atoms in a compound.
- Molecular Formula
The formula that indicates the exact number of each type of atom in a molecule of a compound.
- Mass Percent Composition
The percentage of mass of an element in a compound, calculated based on its total mass.
- Molar Mass
The mass of one mole of a substance typically expressed in g/mol.
Reference links
Supplementary resources to enhance your learning experience.
