Laws of Chemical Combinations
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Law of Conservation of Mass
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll discuss the Law of Conservation of Mass introduced by Antoine Lavoisier. Can anyone tell me what this law states?

It says that mass cannot be created or destroyed in a chemical reaction!

Exactly! This means that in any chemical reaction, the mass of reactants will always equal the mass of products. Can you think of an example of this in everyday life?

When burning wood! The wood seems to disappear but the mass is just converted into gas or ash.

Correct! Remember to always measure your reactants accurately to maintain the balance in reactions. As a memory aid, recall the acronym 'Lavoisier's Law - Matter's Stay!'

I get it! It's like balance beams; both sides must weigh the same.

Great analogy! In summary, Lavoisier's experiments with combustion showed the importance of precise measurement in validating this law.
Law of Definite Proportions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on, what's the Law of Definite Proportions about?

It states that a compound always contains the same proportions of elements by mass.

Right! For example, water is always formed from two hydrogen atoms and one oxygen atom, regardless of the source. Can anyone give me another example?

What about carbon dioxide? It always has the same ratio of carbon to oxygen.

Perfect! To remember this law, think of the phrase 'Definite - Defined Proportions'. In short, all samples of a compound will carry the same elemental composition.

So all sources of water have to be H2O?

Exactly, well done! This law contributes to the reproducibility of chemical reactions.
Law of Multiple Proportions and Gay-Lussac's Law
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss the Law of Multiple Proportions and Gay-Lussac's Law. What do you think Multiple Proportions means?

It means when two elements can form more than one compound, the masses of the one element that combines are ratios of small whole numbers!

Exactly! Like when hydrogen and oxygen form H2O and H2O2. Now, how is Gay-Lussac's Law different regarding volumes?

It relates to the volumes of gases reacting together being in simple whole number ratios at the same temperature and pressure.

Right on! For memory, let's use 'Gases Gather in Integer Groups'. Does everyone understand how these laws complement each other?

Yes, because both laws allow us to predict the outcomes of chemical reactions!

Exactly! Keep these relationships in mind as they are fundamental for understanding stoichiometry.
Avogadro's Law and Summary
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We’ll finish our discussion with Avogadro's Law. What does it state?

It says equal volumes of gases at the same temperature and pressure contain the same number of molecules!

Perfect! Remember, Avogadro's Law is vital for calculating moles and volumes. A mnemonic for this could be 'Avogadro's Arena: All Equal'. Can someone summarize what we've learned about these laws?

We learned about how mass is conserved, the percentages of elements in compounds, whole number ratios in combinations, and gas volumes. It all aligns with atomic theory too!

Well said! In summary, these laws form the foundation for chemical reactions and analyses. Always remember: 'Laws guide us to molecular mastery.'
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The laws of chemical combinations are essential to understanding how substances interact in chemical reactions. This section covers the Law of Conservation of Mass, Law of Definite Proportions, Law of Multiple Proportions, Gay-Lussac's Law of Gaseous Volumes, and Avogadro's Law, illustrating the principles through historical context and practical examples.
Detailed
The Laws of Chemical Combinations present fundamental principles that govern how elements and compounds interact and combine to form new substances.
- Law of Conservation of Mass: Formulated by Antoine Lavoisier in 1789, this law asserts that in any chemical reaction, the total mass of reactants equals the total mass of products. Matter cannot be created or destroyed, highlighting the importance of accurate mass measurements in chemical reactions.
- Law of Definite Proportions: Proposed by Joseph Proust, this law states that a chemical compound contains its constituent elements in fixed, definite proportions by mass regardless of the source or how it was prepared. For example, water (H2O) always consists of hydrogen and oxygen in a 1:8 mass ratio.
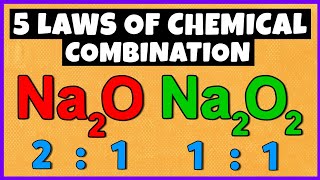
- Law of Multiple Proportions: Introduced by John Dalton, if two elements can form multiple compounds, the ratios of the masses of one element that combine with a fixed mass of the other are in simple whole numbers. For instance, the mass ratios of oxygen in water and hydrogen peroxide (H2O vs H2O2) exemplify this relationship.
- Gay-Lussac's Law of Gaseous Volumes: This law states that when gases react together, the volumes of the reacting gases and the products, if measured at the same temperature and pressure, are in simple whole number ratios.
- Avogadro's Law: Proposed by Amedeo Avogadro, this law relates to the volumes of gases. It posits that equal volumes of gases, at the same temperature and pressure, contain equal numbers of molecules. This significant idea underpins the concept of the mole and the molar volume of ideal gases.
Understanding these laws allows chemists to predict and quantify reactant and product relationships in reactions, forming a foundational aspect of stoichiometry.
Youtube Videos






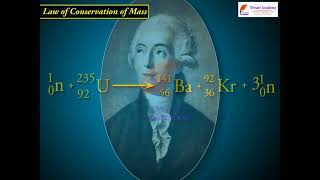
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Law of Conservation of Mass
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This law was put forth by Antoine Lavoisier in 1789. He performed careful experimental studies for combustion reactions and reached the conclusion that in all physical and chemical changes, there is no net change in mass during the process. Hence, he concluded that matter can neither be created nor destroyed. This is called ‘Law of Conservation of Mass’. This law formed the basis for several later developments in chemistry. In fact, this was the result of exact measurement of masses of reactants and products, and carefully planned experiments performed by Lavoisier.
Detailed Explanation
The Law of Conservation of Mass is fundamental in chemistry. It states that in any closed system, the total mass before a chemical reaction must be equal to the total mass after the reaction. This means that atoms are simply rearranged during a chemical reaction; they are not created or destroyed. For example, if you burn a piece of wood, the mass of the ash, gas, and any other products will be equal to the mass of the original piece of wood plus the mass of the oxygen consumed during burning.
Examples & Analogies
Think of a balloon filled with air. Whether the balloon is inflated or deflated, the total amount of air inside (mass) remains the same. If we let some air out of the balloon, we're not creating or destroying the air; we're just moving it in and out of the balloon.
Law of Definite Proportions
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This law was given by a French chemist, Joseph Proust. He stated that a given compound always contains exactly the same proportion of elements by weight. Proust worked with two samples of cupric carbonate — one of which was of natural origin and the other was synthetic. He found that the composition of elements present in it was the same for both the samples.
Detailed Explanation
The Law of Definite Proportions states that a chemical compound will always contain the same proportion of elements by mass. For instance, water (H2O) will always have two hydrogen atoms and one oxygen atom, regardless of where the water comes from, be it the ocean or a glass from the tap. This consistent ratio is what defines the compound.
Examples & Analogies
Imagine baking a cake. No matter where you bake it, if you always use 2 cups of flour and 1 cup of sugar, your cake will have the same basic taste and structure. This is similar to how the same compounds in chemistry maintain their ratios of elements.
Law of Multiple Proportions
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This law was proposed by Dalton in 1803. According to this law, if two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in the ratio of small whole numbers.
Detailed Explanation
The Law of Multiple Proportions tells us that when two elements can form multiple compounds, the masses of one element that combine with a fixed mass of the other element can be expressed as simple whole numbers. For example, carbon and oxygen can form both carbon monoxide (CO) and carbon dioxide (CO2). If you keep the mass of carbon constant, you will see that the mass of oxygen combines in a ratio of 1:2, demonstrating this law.
Examples & Analogies
Consider a set of building blocks. If you have a certain number of blue blocks and a variable number of red blocks, you can build different structures (like CO and CO2), each using whole numbers of red blocks per blue block, showing how combinations can vary while adhering to simple ratios.
Gay Lussac’s Law of Gaseous Volumes
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This law was given by Gay Lussac in 1808. He observed that when gases combine or are produced in a chemical reaction, they do so in a simple ratio by volume, provided all gases are at the same temperature and pressure.
Detailed Explanation
Gay Lussac’s Law states that the volumes of gases involved in a reaction are in a simple ratio when measured at the same temperature and pressure. For example, when hydrogen burns in oxygen to form water vapor, two volumes of hydrogen react with one volume of oxygen to produce two volumes of water vapor. This law helps in understanding the relationships between gaseous reactants and products.
Examples & Analogies
Think about filling balloons with different gases. If you fill up one balloon with 2 liters of hydrogen and another with 1 liter of oxygen at the same temperature and pressure, when they react, the resulting water vapor will be in a balloon that also measures 2 liters, illustrating how the volumes combine in simple ratios.
Avogadro’s Law
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In 1811, Avogadro proposed that equal volumes of all gases at the same temperature and pressure should contain equal number of molecules. Avogadro made a distinction between atoms and molecules, which is quite understandable in present times.
Detailed Explanation
Avogadro’s Law states that equal volumes of gases, under the same conditions of temperature and pressure, have the same number of molecules. This means that one mole of any gas occupies the same volume as one mole of another gas. For instance, 22.4 liters of any ideal gas at standard temperature and pressure (STP) contains Avogadro's number, which is approximately 6.022 × 10²³ molecules.
Examples & Analogies
Consider having two identical balloons: one filled with helium and the other with nitrogen. If both balloons are at the same temperature and pressure and have the same volume, they will contain equally the same number of molecules, despite the difference in gas types, underlining the universality of Avogadro's principle.
Key Concepts
-
Conservation of Mass: Mass is conserved in chemical reactions.
-
Definite Proportions: Compounds have fixed proportions of their constituent elements.
-
Multiple Proportions: Compounds form in whole number ratios.
-
Gaseous Volumes: Gases react in simple volume ratios.
-
Avogadro's Law: Equal volumes of gases at the same conditions contain equal numbers of molecules.
Examples & Applications
Example of Law of Conservation of Mass: When burning a candle, the wax (reactant) transforms into smoke and gas (products) but the mass remains unchanged.
Example of Law of Definite Proportions: Water (H2O) always has the same ratio of hydrogen to oxygen (2:1) regardless of the source.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the dance of mass, as it moves with grace, / No loss, no gain, just a constant face.
Stories
Imagine a chef who uses the same recipe for cake, each ingredient must match for the cake to take. Just like compounds form together, keeping proportions whole in forever.
Memory Tools
Remember the acronym 'CME' for Conservation of Mass, Definite Proportions, and Multiple Proportions.
Acronyms
Use 'VGA' for Gay-Lussac's perspective
Volumes of Gases Always equal numbers.
Flash Cards
Glossary
- Law of Conservation of Mass
A principle that states that the total mass of reactants in a chemical reaction must equal the total mass of products.
- Law of Definite Proportions
A law stating that a chemical compound contains its constituent elements in fixed ratio by mass.
- Law of Multiple Proportions
A law stating that when two elements form more than one compound, the masses of one element that combine with a fixed mass of the other are in the ratio of small whole numbers.
- GayLussac's Law
A principle that states that when gases react together, they do so in simple whole number ratios by volume, provided temperature and pressure are constant.
- Avogadro's Law
A law stating that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules.
Reference links
Supplementary resources to enhance your learning experience.
