Scientific Notation
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Scientific Notation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome class! Today we’re learning about scientific notation, which is crucial in chemistry because it allows us to express very large or small numbers concisely. Why do you think we need to express such numbers differently?

Because numbers can get very big or very small, and it’s hard to handle so many zeros!

Exactly! For example, instead of writing 602,200,000,000,000,000,000,000, we can write it as 6.022 x 10^23, which is much easier to read and work with. Can anyone tell me a situation in chemistry where you’d encounter very small or very large numbers?

When we're talking about the number of atoms in a molecule!

Great example! Let's also consider the very small mass of a hydrogen atom, approximately 0.00000000000000000000000166 g, which can be expressed as 1.66 x 10^-24 g.
Converting to Scientific Notation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's practice converting numbers into scientific notation. Who can tell me how we convert 5672 into scientific notation?

We move the decimal to the left so it becomes 5.672, then we count how many places we moved it, which is 3, so it’s 5.672 x 10^3!

Correct! For decimals, like 0.0045, we move the decimal to the right to get 4.5, and since we moved it 3 places, it's 4.5 x 10^-3. Can anyone see the pattern here?

It depends on whether the number is large or small, and we adjust the exponent accordingly!

That's right! Moving left increases the exponent, while moving right decreases it.
Operations with Scientific Notation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's learn how to perform operations with numbers in scientific notation. For addition, you need the exponents to be the same. If I have 3.0 x 10^4 and 4.2 x 10^4, how would we add them together?

You would just add 3.0 and 4.2, since they both have the same exponent, so it’s 7.2 x 10^4.

Perfect! Now for multiplication, you multiply the coefficients and add the exponents. So if we have 2 x 10^3 and 3 x 10^2, what do we get?

That would be 6 x 10^5, because 3 plus 2 is 5!

Exactly! Let’s summarize what we covered today. Scientific notation allows us to express large and small numbers, convert numbers into this format, and conduct arithmetic operations while ensuring accuracy.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses scientific notation, explaining its utility in handling values in chemistry, the method to convert standard numerical values into scientific format, and the rules for performing mathematical operations with numbers in scientific notation. It emphasizes its importance for expressing both extremely large and small quantities in a standardized way.
Detailed
Scientific Notation
Scientific notation simplifies calculations involving extremely large or small quantities by expressing them in the form of N × 10^n, where N is a number between 1 and 10, and n is an integer. This notation is particularly useful in chemistry where atoms, molecules, or constants such as the speed of light are expressed in rates that can span a wide range of magnitudes.
For instance, the number of molecules in 2 grams of hydrogen gas can be represented as 6.022 × 10²³ molecules. Conversely, the mass of a hydrogen atom can be expressed as 1.66 × 10⁻²⁴ grams.
When performing mathematical operations on numbers in scientific notation, it is crucial to adhere to specific rules for addition, subtraction, multiplication, and division. For addition, the exponents must be the same before combining the coefficients. For multiplication, the coefficients are multiplied, and exponents are added (a × 10^m) × (b × 10^n) = ab × 10^(m+n). Scientific notation enhances mathematical clarity, allowing chemists to handle an array of values more conveniently.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Scientific Notation
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As chemistry is the study of atoms and molecules, which have extremely low masses and are present in extremely large numbers, a chemist has to deal with numbers as large as 602,200,000,000,000,000,000,000 for the molecules of 2 g of hydrogen gas or as small as 0.00000000000000000000000166 g mass of a H atom. Similarly, other constants such as Planck’s constant, speed of light, charges on particles, etc., involve numbers of the above magnitude.
Detailed Explanation
In chemistry, we often encounter measurements that either have very large values, such as the number of atoms in a sample, or very small values, such as the mass of a single atom. To avoid writing out long numbers with many zeros, scientists use a method called scientific notation. This notation allows us to express these large and small numbers in a compact format, making calculations easier and clearer.
Examples & Analogies
Think of scientific notation like a zip code. Just as a zip code provides a quick way to locate a place without writing out the entire address, scientific notation allows scientists to quickly understand and work with very large or small numbers without listing all the zeros.
Form of Scientific Notation
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
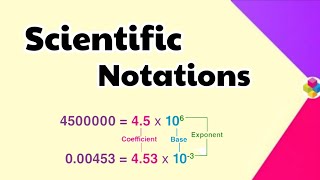
This problem is solved by using scientific notation for such numbers, i.e., exponential notation in which any number can be represented in the form N × 10^n, where n is an exponent having positive or negative values and N is a number (called digit term) which varies between 1.000... and 9.999....
Detailed Explanation
Scientific notation has a very specific format: it consists of a digit term followed by a power of ten. The digit term (N) must be a number greater than or equal to 1 and less than 10, while the exponent (n) indicates how many places the decimal point has been moved. A positive exponent means we are dealing with a large number, and a negative exponent indicates a small number.
Examples & Analogies
Consider N as the main actor of a play, representing the value you want to express, and n as the stage on which the actor performs, showing the scale of that number. For example, if N is '3', then n as '10^3' means the actor is putting on a large production. If N is '0.003', then moving n to '10^(-3)' shows it's a small, intimate performance.
Converting Numbers to Scientific Notation
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thus, we can write 232.508 as 2.32508 × 10^2 in scientific notation. Note that while writing it, the decimal had to be moved to the left by two places and same is the exponent (2) of 10 in the scientific notation. Similarly, 0.00016 can be written as 1.6 × 10^(-4).
Detailed Explanation
To convert a regular number into scientific notation, you identify the first non-zero digit, place the decimal point after this digit, and count how many places you moved the decimal. If you moved it to the left, the exponent will be positive; if you moved it to the right, the exponent will be negative. For example, for the number 232.508, moving the decimal to the left two times gives us the scientific notation of 2.32508 × 10^2.
Examples & Analogies
Imagine you're packing boxes for a move. If a large box can only be moved by a truck (large exponent), that's like moving the decimal to the left (large number). If you need to squeeze something small like a toy into your pocket (small exponent), that's similar to moving the decimal to the right (a small number).
Performing Operations in Scientific Notation
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
While performing mathematical operations on numbers expressed in scientific notations, the following points are to be kept in mind.
Detailed Explanation
When working with numbers in scientific notation, operations like addition, subtraction, multiplication, and division follow specific rules. For multiplication and division, you work with the coefficients separately from the powers of ten, and for addition and subtraction, you must first match the exponents before proceeding with the digit terms.
Examples & Analogies
Think of scientific notation like a recipe. When you multiply ingredients, you handle the amounts (coefficients) separately before combining them, similar to how you deal with the powers of ten. When you add ingredients (like flavors), you must ensure you're using the same measurement (exponent), like converting all your cups to the same size before combining them.
Key Concepts
-
Scientific Notation: A system for expressing very large or very small numbers.
-
Exponents: Indicate the magnitude by which the base number is multiplied.
-
Coefficient: The part of a number in scientific notation before multiplying by 10.
-
Significant Figures: Indicate the precision of measured quantities.
Examples & Applications
To express 5000 in scientific notation, write it as 5.0 x 10^3.
The mass of an electron is approximately 9.11 x 10^-28 grams.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When writing numbers short and sweet, Keep the digits neat, make it discrete. Move the decimal, count the score, Powers of ten, just one more!
Stories
Imagine you're packing your backpack for a long trip. Instead of writing the full weight of each item, you decide to jot down how many times heavier it is than 1 kilogram. This way, your notes are light and easy to manage, just like scientific notation!
Memory Tools
For addition in scientific notation, think ADA: Align Decimal, Add coefficients, Adjust the exponent if needed.
Acronyms
SIS
Scientific In Simplification – a reminder that scientific notation simplifies understanding complex numbers.
Flash Cards
Glossary
- Scientific Notation
A method of expressing numbers in the form N × 10^n, where N is between 1 and 10, and n is an integer.
- Exponents
Numbers that indicate how many times a base number is multiplied by itself.
- Coefficient
The numerical factor in a term.
- Significant Figures
Digits in a number that carry meaningful information about its precision.
Reference links
Supplementary resources to enhance your learning experience.
