On the basis of energy bands
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Energy Bands
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

When atoms come close during the formation of solids, their outermost orbitals overlap. This overlap affects how electrons behave in solids. Let's explore how this creates energy bands.

What are these energy bands you mentioned?

Great question! Energy bands are ranges of energy that electrons can occupy. There are mainly two types: the valence band and the conduction band.

Can you explain the difference between these two bands?

Of course! The valence band is where the valence electrons reside. It is usually filled at low temperatures. The conduction band is higher in energy and, ideally, empty when the material is at absolute zero.

So the conduction band affects how well a material can conduct electricity?

Exactly! The ability of electrons to move into the conduction band is what determines a material's conductivity.

Are all materials the same in regards to these bands?

Not at all! That's where classification comes in: metals have overlapping bands allowing high conductivity, insulators have a significant gap preventing conduction, and semiconductors have a small gap allowing them to conduct under certain conditions.
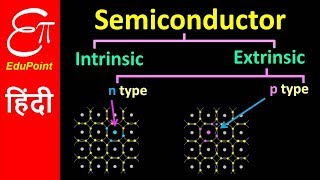
Classification of Semiconductors and Conductors
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s dive deeper into how energy gaps classify materials. What could happen if the conduction band is empty?

It sounds like that would mean the material isn't conducting electricity?

Exactly! If the conduction band is empty and there's a large energy gap, the material will behave like an insulator.

But what about semiconductors? How do they work?

Great observation! In semiconductors, the energy gap is smaller, usually less than 3 eV. This allows some electrons to jump into the conduction band from the valence band under the right conditions, such as thermal energy.

What kind of influences can help electrons jump that gap?

External energy sources like heat or light can provide enough energy to help electrons move into the conduction band, making semiconductors conductive.

So, does that mean we can also control their conductivity?

Exactly! This characteristic makes semiconductors extremely useful for electronic devices.
Practical Implications of Energy Bands
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s relate this back to technology. Why do you think understanding energy bands is crucial for electronics?

I guess it's essential for designing components like diodes and transistors?

Absolutely spot on! Devices like transistors utilize the controlled conductivity of semiconductors, which is rooted in their energy band structure.

Could you elaborate on how we've moved from vacuum tubes to transistors?

Sure! Vacuum tubes were less efficient and bulky. The development of semiconductors not only reduced size but also improved power efficiency and reliability in electronic devices.

So energy bands really play a role in shaping our modern electronics?

Indeed! They define how materials behave under electrical forces, which is fundamental in semiconductor technology.

Can we say our smartphones are benefiting from these principles?

Yes, every electronic device you use leverages these principles, showcasing the importance of understanding energy bands.
Energy Band Gap Scenarios
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss the various scenarios depicted in the section. What happens in metals?

There is no energy gap between the bands, right?

Correct! This allows free movement of electrons, leading to high conductivity. Now, what about insulators?

Insulators have a large gap, which means electrons can't jump to the conduction band easily.

Right! And this leads to their high resistance. In semiconductors, energy gaps are smaller. Can anyone tell me the energy gap range for semiconductors?

It's generally less than 3 eV?

Exactly! This small gap is what allows semiconductors to be manipulated for various applications.

What is the significance of thermal excitation in this context?

Thermal excitation is crucial as it allows some electrons to gain enough energy to jump the gap and enhance conductivity.
Summary and Application
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To summarize, we learned that energy bands play a vital role in determining the electrical properties of materials. Can anyone recall how each type of material is classified?

Metals have overlapping bands, insulators have a large gap, and semiconductors have a small gap.

Very well! And why is this knowledge essential in electronics?

It helps us develop more efficient components and understand how to control conductivity!

Precisely! This section builds a foundation for understanding semiconductor devices, which are pivotal in modern technology.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explores how energy bands are formed when atoms come together to create a solid and how these bands determine the electrical properties of the material, including scenarios for metals, insulators, and semiconductors. It emphasizes the gap between the valence band and conduction band and its influence on material behavior.
Detailed
Overview
In solid-state physics, as atoms bond to form solids, the atomic orbitals of adjacent atoms overlap, resulting in the formation of energy bands.
Energy Bands
Energy levels of electrons in solids are represented as continuous energy bands instead of discrete levels. The two primary bands are:
- Valence Band: Contains the energy levels of the valence electrons and is fully occupied at absolute zero.
- Conduction Band: Lies above the valence band, typically unoccupied at absolute zero.
Material Classification
- Metals: Characterized by overlapping energy bands, allowing electrons to flow freely, resulting in high conductivity.
- Insulators: Have a significant energy gap between the valence and conduction bands (usually >3 eV), which prevents any electron mobility, leading to high resistance.
- Semiconductors: Exhibit a smaller energy gap (<3 eV) enabling some electrons to jump from the valence band to the conduction band under certain conditions (like thermal excitation at room temperature). This property allows them to conduct electricity under specific scenarios.
Conclusion
This understanding of energy bands is critical for developing semiconductor devices, as it outlines the theoretical foundation for electron movement and electrical conductivity in materials.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Energy Levels in Solids
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
According to the Bohr atomic model, in an isolated atom the energy of any of its electrons is decided by the orbit in which it revolves. But when the atoms come together to form a solid they are close to each other. So the outer orbits of electrons from neighbouring atoms would come very close or could even overlap. This would make the nature of electron motion in a solid very different from that in an isolated atom.
Detailed Explanation
In an isolated atom, electrons exist in specific energy levels defined by their orbits. However, when atoms bond together to form a solid, the proximity of their outer orbits causes these energy levels to interact, leading to overlapping. This overlap changes how electrons behave in a solid as compared to isolated atoms. Instead of discrete energy levels, electrons in solids experience a continuum of energy levels. This phenomenon forms the basis of the concept of energy bands in solids.
Examples & Analogies
Think of atoms as people on a dance floor. When they are alone (isolated atoms), they can only move in their own limited space (their orbit). But when they get together at a crowded party (solid), they have to share space and can move in ways that are not possible when they are alone. This change in movement dynamics reflects how energy levels combine into bands in solids.
Energy Bands: Valence and Conduction Bands
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Inside the crystal each electron has a unique position and no two electrons see exactly the same pattern of surrounding charges. Because of this, each electron will have a different energy level. These different energy levels with continuous energy variation form what are called energy bands. The energy band which includes the energy levels of the valence electrons is called the valence band. The energy band above the valence band is called the conduction band.
Detailed Explanation
In a crystal, every electron occupies a specific position based on the surrounding charges from neighboring atoms. Because the environment surrounding each electron varies slightly due to the presence of other charged particles, each electron can inhabit a slightly different energy level. Collectively, these energy levels merge to create 'energy bands'. The valence band is a lower band where electrons are usually present, and the conduction band is the higher band where electrons can move freely when sufficient energy is provided to cross the gap between these bands.
Examples & Analogies
Imagine a library (the solid) with multiple floors (energy bands). The bookshelves on the ground floor represent the valence band where all the popular books are stored. The upper floors represent the conduction band where only a few selected books can be accessed. If someone wants to go upstairs (gain energy), they need to take the stairs (energy band gap) to reach the upper floors.
Behavior of Electrons in Different Materials
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
With no external energy, all the valence electrons will reside in the valence band. If the lowest level in the conduction band happens to be lower than the highest level of the valence band, the electrons from the valence band can easily move into the conduction band. Normally the conduction band is empty. But when it overlaps on the valence band, electrons can move freely into it. This is the case with metallic conductors.
Detailed Explanation
In most materials, under normal conditions, valence electrons occupy the valence band. If the conduction band is either overlapping with or very close to the valence band, then electrons can be easily excited into the conduction band where they can contribute to electrical conduction. In metals, the conduction band overlaps the valence band, allowing electrons to flow freely, resulting in high electrical conductivity.
Examples & Analogies
Picture a road where cars (electrons) are parked (valence band) in a parking lot. If the road (conduction band) is right next to the parking lot and the gates (energy gap) are wide open, cars can easily drive out onto the road. This free movement of cars symbolizes the easy flow of electricity in metal conductors.
Insulators and Band Gaps
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If there is some gap between the conduction band and the valence band, electrons in the valence band all remain bound and no free electrons are available in the conduction band. This makes the material an insulator. But some of the electrons from the valence band may gain external energy to cross the gap between the conduction band and the valence band. Then these electrons will move into the conduction band.
Detailed Explanation
Materials that have a significant energy gap between the valence band and the conduction band do not permit electrons to flow freely, making them good insulators. The gaps can be so large that electrons cannot gain enough energy from thermal or light sources to jump into the conduction band. However, if the energy is sufficient, some electrons might acquire the required energy, perform the 'jump' over the barrier, and contribute to conductivity.
Examples & Analogies
Consider a high barrier wall blocking access between two areas (valence and conduction bands). If the wall is extremely tall (large band gap), people (electrons) can't climb over easily and hence remain in their respective areas (insulators). But if they get a boost (external energy), they might be able to jump over and move freely in the other area.
Semiconductors and Energy Gaps
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Here a finite but small band gap (E < 3 eV) exists. Because of the small band gap, at room temperature some electrons from the valence band can acquire enough energy to cross the energy gap and enter the conduction band. These electrons (though small in numbers) can move in the conduction band. Hence, the resistance of semiconductors is not as high as that of the insulators.
Detailed Explanation
Semiconductors possess a relatively small band gap, allowing some thermal energy at room temperature to promote electrons from the valence band into the conduction band. This means that there will always be a few free electrons available to conduct electricity, making semiconductors less resistive than insulators, which have no free conduction electrons.
Examples & Analogies
Imagine a crowded but accessible bridge (the small band gap in semiconductors) on a busy street where some people are able to get onto the bridge to cross over (electrons moving to conduction band), unlike in a scenario where the bridge is completely barricaded (large band gap in insulators). Even if only a few succeed in crossing, it still allows for a semblance of movement that wouldn't be possible otherwise.
Key Concepts
-
Energy Bands: Ranges of energy levels derived from overlapping atomic orbitals in solids.
-
Valence Band: The band where valence electrons exist at absolute zero.
-
Conduction Band: An energy band that becomes occupied by electrons if sufficient energy is provided.
-
Energy Band Gap: The energy difference between the valence and conduction bands that determines the electrical conductivity of a material.
-
Classification of Materials: Metals have overlapping bands, insulators have large gaps, and semiconductors have small gaps.
Examples & Applications
Metals like copper and silver have overlapping energy bands, allowing for high conductivity.
Insulators like rubber have large energy band gaps (>3 eV), preventing electron flow and making them poor conductors.
Silicon and germanium are common semiconductors with smaller band gaps, allowing them to conduct electricity under the right conditions.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In metals, bands overlap, electrons do tap; insulators' gap is large, no charge to charge.
Stories
Imagine a city where some roads are wide (metals), making traffic flow easily, while others are blocked (insulators) with only a few narrow paths (semiconductors) allowing cars to move under certain conditions.
Memory Tools
M-S-I: Metals, Semiconductors, Insulators – think of their positions in conductivity.
Acronyms
EBC (Energy Band Classification) - helps to remember Energy Bands, Band Gap, and their Conductivity characteristics.
Flash Cards
Glossary
- Energy Band
A range of energy levels that electrons can occupy in a solid; primarily consists of valence and conduction bands.
- Valence Band
The energy band that contains the energy levels of valence electrons.
- Conduction Band
The energy band above the valence band, which is typically empty at low temperatures and allows for electron mobility.
- Energy Band Gap
The energy difference between the top of the valence band and the bottom of the conduction band, influencing a material's conductivity.
- Metal
A material with overlapping energy bands, allowing for high electrical conductivity.
- Insulator
A material with a large energy gap, leading to very low electrical conductivity.
- Semiconductor
A material with a small energy gap that allows for variable conductivity based on external conditions.
Reference links
Supplementary resources to enhance your learning experience.
