Capillarity Height
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Capillarity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome everyone! Today we will explore the fascinating world of capillary action. Can anyone tell me what capillarity means?

Isn't it about how liquids can rise in small tubes?

Great observation! Yes, capillarity is the ability of a liquid to flow in narrow spaces without external forces. This plays a crucial role in various fields, especially in civil engineering.

How does the surface tension come into play here?

Excellent question! Surface tension is one of the critical factors in determining the height a liquid will rise in a capillary tube. It pulls the liquid upwards against gravity.

So the narrower the tube, the higher the liquid rises?

Precisely! The diameter of the tube plays a significant role in the capillary height. The smaller the diameter, the higher the liquid can rise.

What is the formula to calculate this height?

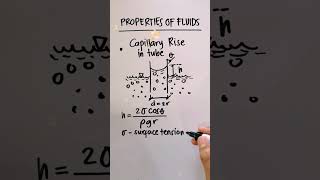
The formula is $$ h = \frac{4\sigma \cos(\theta)}{\rho g d} $$ where σ is the surface tension, θ is the contact angle, ρ is the liquid density, g is the acceleration due to gravity, and d is the diameter of the tube.

To summarize, capillarity is influenced by surface tension, tube diameter, and the contact angle. Understanding these relationships is critical in fluid mechanics.
Deriving Capillarity Height Formula
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we have the formula, let’s break down how we derive it. Who remembers why surface tension is so crucial?

It creates a force that can hold the water molecules together?

Exactly! This force acts at the liquid-air interface and contributes to the upward movement of the liquid. Let's explore the mathematical relationships.

What happens if the contact angle changes?

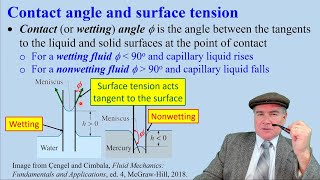
Good point! If the angle θ increases, the cosine decreases, which means the height *h* also decreases, indicating poor wetting. A perfect wetting situation has a contact angle of 0 degrees.

So does this mean non-wetting liquids won't rise at all?

Correct! Such as in the case of mercury in glass. The liquid wouldn’t rise due to high contact angle.

In summary, capillarity combines surface tension, contact angle, and tube dimensions, influencing fluid behavior in confined conditions.
Applications of Capillarity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss some applications of capillary action. Why do you think it’s important in engineering?

It helps understand how plants draw water from the ground.

Exactly! Capillarity is essential in soil moisture retention and determines how plants transport water.

What about in construction?

Capillarity affects how fluids behave in porous materials, which can impact the durability and stability of structures.

Is there an example in everyday life?

Absolutely! A simple example is how a paper towel absorbs liquid. The capillary action draws the liquid upward.

To wrap up, understanding capillarity informs us about fluid dynamics in various natural and engineered systems.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section delves into capillarity height in fluid mechanics, detailing the equations governing surface tension, diameter of capillary tubes, and the angle of contact, while relating these concepts to hydrostatic principles and practical applications.
Detailed
Detailed Summary
In fluid mechanics, capillarity refers to the ability of a liquid to flow in narrow spaces without the assistance of external forces—essentially how high a liquid can rise in a narrow tube, known as a capillary tube. The capillary height, denoted as h, can be determined using the formula:
$$ h = \frac{4\sigma \cos(\theta)}{\rho g d} $$
where:
- σ = surface tension force,
- θ = angle of contact,
- ρ = liquid density,
- g = acceleration due to gravity,
- d = diameter of the capillary tube.
This section emphasizes the relationship between surface tension, the geometry of the liquid's container, and the resultant height of liquid in the tube. Understanding capillarity is vital since it impacts numerous processes in hydraulic and civil engineering, including soil moisture retention and fluid transport in plant systems. Additionally, the section ties it into hydrostatics and the implications of liquid behavior at rest, leading to deeper insights into fluid mechanics.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Capillarity Height
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Similar way also we derived the capillarity height in terms of the diameter of the capillarity tube.
Capillary height
h = (4 * σ * cos(θ)) / (ρ * g * d)
Where:
- h = capillary height
- σ = surface tension force
- θ = angle of contact
- d = diameter
Detailed Explanation
Capillarity height is the height to which a liquid can rise or fall in a narrow tube due to surface tension. The equation provided expresses this height (h) in terms of various factors:
1. Surface tension (σ) creates a force that pulls the liquid upward.
2. The angle of contact (θ) indicates how wet the liquid is against the surface of the tube. A smaller angle improves capillary action.
3. The diameter (d) of the tube is important; a smaller diameter results in a greater capillary height because the surface area relative to the volume increases, enhancing the effect of surface tension.
Examples & Analogies
Imagine a thin straw in a glass of water. When you place the straw in the water, the water rises up the straw due to capillarity. The narrower the straw is, the higher the water will rise. This phenomenon can be compared to a tiny sponge soaking up water. The narrower the space within the sponge, the more water it can absorb due to capillary action.
Surface Tension and Pressure Difference
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
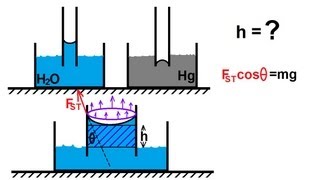
The surface tension force when we equate with a pressure difference and the area where it is acting it the force, both the side the force equating what you have done it the force the net force acting on this part is taking care of the force due to the surface tensions.
Detailed Explanation
The concept of surface tension leads to a pressure difference across the liquid's surface in a capillary tube. When liquid rises due to surface tension, the upward pressure (from the liquid's internal forces) needs to balance the downward pressure caused by the weight of the liquid column. This equilibrium determines the height to which the liquid can rise. The balance of forces involves setting the force generated by surface tension equal to the hydrostatic pressure difference created by the height of the liquid column.
Examples & Analogies
Think about a water droplet on a leaf: the droplet tends to hold its shape due to surface tension. If you have a tiny straw and place it in the droplet, the water inside the straw will rise up until the weight of the water column inside the straw balances the force from the surface tension pulling it up and the weight of the water itself.
Pressure Distribution in Static Fluids
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As you know very basic things, when you consider z as a at the free surface level is zero. As z increases in the downwards the pressure will be P = ρgh.
Detailed Explanation
In a static fluid, the pressure at a given depth (z) can be calculated using the formula P = ρgh, where:
- P is the pressure at depth z,
- ρ (rho) is the density of the fluid,
- g is the acceleration due to gravity,
- h is the height of the fluid column above that point. This relationship means that as you go deeper into a fluid, the pressure increases linearly with depth.
Examples & Analogies
It's like diving into a swimming pool—if you're underwater, you can feel the pressure increasing as you go deeper. At the surface, your ears might feel normal, but as you descend, they may pop due to the increasing pressure, demonstrating how pressure builds with depth.
Equilibrium of Forces and Capillarity
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
And very simple, what will be the pressure distribution in a fluid which is at the rest the static fluid will be the weight of the fluid divide by the area.
Detailed Explanation
In a static fluid, to find the equilibrium state, it's essential to consider how the forces (like pressure and buoyancy) balance each other. In capillarity, the weight of the liquid column it counteracts the upward force due to surface tension. Essentially, when calculating pressures in a column of liquid, the pressure is determined by the weight of the liquid above divided by its cross-sectional area.
Examples & Analogies
Imagine a tall tower of blocks. Each block adds weight, but the total weight is distributed over the area of the base. If you were to push down on the top of the blocks, the pressure at the bottom felt by the base would depend on how many blocks were stacked above and the area they cover.
Summary of Capillarity Concepts
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let me repeat these things that very simple things as we discuss the Newton laws of viscosity which establish the relationship between shear stress and the velocity gradient. Similar way we derive the capillarity height will be a functions of contact angles, surface tensions, and the diameter of the capillarity tube.
Detailed Explanation
In summary, capillarity height is derived based on laws of physics-related forces acting on a fluid surface, specifically addressing the balance of surface tension, liquid cohesion, and density of the fluid. The established relationships allow for predicting how high a fluid will rise in a capillary tube based on its properties and the tube's characteristics.
Examples & Analogies
If you've ever seen how a paper towel absorbs water, that's capillary action at work! The tiny spaces in the towel draw water upwards, demonstrating how surfaces allow liquids to climb when they are sufficiently narrow. This is the same principle applied in various engineering mechanisms, like how ink moves in a fountain pen.
Key Concepts
-
Capillarity: The ability of liquids to rise in narrow tubes due to surface tension.
-
Surface Tension (σ): The force that causes the liquid surface to contract, affecting capillary rise.
-
Contact Angle (θ): The angle between the liquid and a solid surface that influences how well the liquid wets the surface.
-
Influence of Diameter (d): The smaller the diameter of the capillary tube, the higher the liquid can rise due to increased surface tension influence.
Examples & Applications
The rise of water in a thin straw demonstrates capillary action as the liquid travels against gravity.
In plants, water moves up from the roots through narrow xylem tubes utilizing capillary action to reach the leaves.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a tube so small, water climbs tall, surface tension helps, against gravity, after all.
Stories
Imagine a tiny ladder for water, where surface tension is the hand that lifts it up, defying gravity in a thin straw.
Memory Tools
Remember: S = Surface Tension, C = Capillary Height, D = Diameter, A = Angle: S-C-D-A forms the factors!
Acronyms
Use CAPE - Contact angle, Area, Pressure, and Elevation to remember key factors of capillarity.
Flash Cards
Glossary
- Capillarity
The ability of a liquid to flow in narrow spaces without an external force.
- Surface Tension
The cohesive force at the liquid's surface, causing it to behave like an elastic sheet.
- Contact Angle
The angle formed between the liquid and the surface with which it is in contact.
- Hydrostatics
The branch of physics that studies fluids at rest.
- Diameter of Capillary Tube
The width of a capillary tube that affects how high a liquid can rise.
- Density (ρ)
The mass per unit volume of a substance, crucial for calculating fluid behavior.
Reference links
Supplementary resources to enhance your learning experience.
