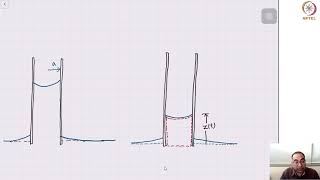
Capillary Rise in Annular Tubes
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Capillary Action
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are diving into the fascinating phenomenon of capillary action. Can anyone tell me what capillary action is?

Isn't it the ability of a liquid to rise in a narrow space, like in a tube?

Exactly! Capillary rise occurs due to the balance between gravitational forces and the forces acting at the liquid's surface, primarily surface tension. Remember the acronym 'SAC' for Surface tension, Adhesion, and Cohesion. These three factors drive capillarity. Now, can anyone explain how surface tension plays a role?

Surface tension allows the liquid to climb up the walls of the tube because it creates a force that pulls the liquid up.

Correct! It's crucial to note how that force varies with the diameter of the tube, especially in annular tubes. Let's summarize: Capillarity occurs due to surface tension, and it can be influenced by both the tube’s diameter and the angle of contact.
Deriving Capillary Rise Formula
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's derive the formula for capillary rise in annular tubes. Who remembers how we set up the forces acting on the liquid in the tube?

We start with the balance of the weight of the liquid column and the upward force due to surface tension!

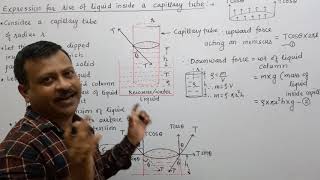
Right! If we let \(h\) be the height of the liquid column, and using the contact angle \(\phi\), we can establish that: \( h = \frac{4 \sigma \cos(\phi)}{\rho g (D - d)} \). Can someone explain why each term in this equation matters?

The numerator represents the upward force due to surface tension, while the denominator is the weight of the liquid, which depends on density \(\rho\), gravity \(g\), and the effective area between the diameters.

Excellent! So, the more significant the surface tension or the smaller the gap, the higher the liquid will rise.
Applications of Capillary Action
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone provide an application for capillary action in real life?

Plants use capillary action to draw water from the soil!

That's a great example! This principle is pivotal in many fields, from agriculture to engineering materials. In materials science, especially, understanding how fluids behave in small spaces can lead to innovations. How might capillary action be a concern in engineering?

If water gets into small gaps in structures, it could affect insulation or cause damage over time.

Exactly! So remember the importance of capillary action: it not only influences how plants function but also impacts engineering design and material selection.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Capillary rise in annular tubes is influenced by surface tension, the angle of contact, and the diameters of the inner and outer tubes. Understanding these relationships is critical in applications such as fluid transport and materials science.
Detailed
Capillary Rise in Annular Tubes
The concept of capillary rise is fundamental in fluid mechanics, particularly in annular tubes where liquids can be drawn upwards due to surface tension. The capillary height
(\(h\)) can be expressed in terms of the surface tension force and the geometrical factors of the annulus.
Key Factors Influencing Capillary Rise:
- Surface Tension (\(\sigma\)): This is a force that acts on the surface of a liquid, influencing its ability to rise against gravity.
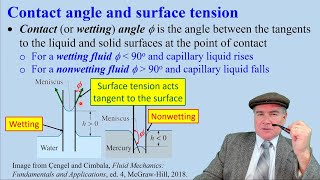
- Angle of Contact (\(\phi\)): The angle formed at the interface of the liquid and the surface of the tube. This affects the adhesion between the liquid and the surface of the tube.
- Inner and Outer Diameters (\(d\) and \(D\)): The diameters of the annular tube dictate the geometry through which the liquid moves, influencing the height to which the liquid can rise.
In equilibrium, the weight of the liquid column is balanced by the forces due to surface tension. Capillary action is essential for several natural and industrial processes, such as the movement of water in plants and in the design of various engineering systems.
Youtube Videos





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Overview of Capillarity
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Two coaxial glass tubes forming an annular with a small gap are immersed in clear water. The inner and outer diameters are small d and the capital D respectively.
Detailed Explanation
This statement introduces the concept of capillarity, which refers to the ability of a liquid to flow in narrow spaces without the assistance of external forces. In this case, we have two coaxial tubes, which means one tube is inside the other, creating a thin space, or annulus, between them. The diameters of these tubes are defined as 'd' for the inner tube and 'D' for the outer tube. The ability of water to rise in this space is a demonstration of capillary action.
Examples & Analogies
Think of this situation like using a straw. When you place a straw in a drink, the liquid rises within the straw without any external force. Similarly, water rises between two coaxial tubes due to adhesive forces between the water and the glass.
Understanding Surface Tension
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
What is capillary rise of water in the annular if the sigma is the surface tension of the water in contact with air and phi is the angle of contact between water and glass tube?
Detailed Explanation
This section highlights the factors that influence capillary rise. The symbol 'sigma' represents the surface tension of the water, which is a force that acts at the surface of a liquid. It is what allows the water to rise in the narrow gap between the tubes. The 'phi' represents the angle of contact between the water and the glass tube, which affects how water interacts with the surface. A smaller angle indicates better adhesion of water to the glass, leading to a higher capillary rise.
Examples & Analogies
Imagine placing a piece of paper towel into a glass of water. You will see the water rise up the towel due to the surface tension. The angle at which the water meets the towel also determines how high the water climbs. This relationship can help us understand the mechanics behind the movement of water in the annular tubes.
Conditions for Capillary Rise
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The fluid outside since is it at the atmospheric pressures, the fluid which is inside in this capillary tube also will be the atmospheric pressures.
Detailed Explanation
In capillarity, the pressure of the fluid outside of the tubes is equal to the atmospheric pressure. This condition is crucial because the height to which water can rise depends on balancing the weight of the water column against the forces exerted by surface tension. The equilibrium happens when the weight of the water in the annular tube is balanced by the upward force of surface tension acting at the liquid's surface.
Examples & Analogies
Think of balancing a variety of weights on a seesaw. If one side gets too heavy (the weight of the water in the tube), it will tip. Similarly, the weight of the liquid must be perfectly counteracted by the forces from the surface tension for the water to remain at a certain height within the tubes.
Effect of Adhesion
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Due to adhesion of water to wall of vessel, the meniscus turns upwards making an angle of phi.
Detailed Explanation
This describes how adhesive forces between the water and the glass wall of the tubes cause the water to rise and create a curved surface, known as the meniscus. The angle 'phi' refers to this curvature, which is a visual representation of how much the water is being pulled up by the walls of the tube due to adhesion. This upward curve is key to understanding how high the liquid will rise in the annular space.
Examples & Analogies
When you fill a glass with water, you'll notice that the surface of the water is slightly higher around the edges. This is similar to the way the water rises along the inner walls of the glass because the water molecules are attracted to the glass, which illustrates adhesion.
Key Concepts
-
Capillary Rise: The movement of liquid within a narrow space due to surface tension.
-
Influence of Surface Tension: Surface tension is critical for determining how high a liquid will rise.
-
Importance of Tube Geometry: The inner and outer diameters of annular tubes affect the capillary action.
Examples & Applications
When a thin straw is placed in a glass of water, the water rises inside the straw due to capillary action.
In plants, capillary action helps transport water and nutrients from the roots to the leaves.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a tube that's narrow and small,
Stories
Imagine a tiny gardener; he pours water into a narrow straw, and to his surprise, the water rises all the way to the top, guided by the invisible forces of nature.
Memory Tools
Remember the acronym 'SAC' for Surface tension, Adhesion, and Cohesion in capillary action.
Acronyms
H.A.W
Height equals the Area and Weight balance in capillary rise.
Flash Cards
Glossary
- Capillarity
The capability of liquid to flow in narrow spaces without the assistance of external forces.
- Surface Tension
The tension of the surface film of a liquid due to the attraction of the particles in the surface layer.
- Angle of Contact
The angle formed at the interface between the liquid and the solid surface.
- Annular Tube
A tube-like structure formed by two concentric tubes, resulting in an annular space.
- Hydrostatic Pressure
The pressure exerted by a fluid at equilibrium due to the force of gravity.
Reference links
Supplementary resources to enhance your learning experience.
