BOND PARAMETERS
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Bond Length
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To start our discussion today, let’s delve into the concept of bond length. Can anyone tell me what bond length signifies?

Is it the distance between the two bonded atoms?

Exactly! Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms. This distance varies with different types of bonds. What do you think happens to the bond length when we compare single, double, and triple bonds?

I think single bonds would be longer than double bonds, and double bonds would be longer than triple bonds.

Correct! As the bond order increases, the bond length decreases. So, single bonds are the longest, while triple bonds are the shortest. Remember, this relationship can help us understand the strength and stability of molecules. A good mnemonic to remember is 'Silly Dogs Think' for Single, Double, and Triple bonds respectively.

Can you give us an example of bond lengths in a molecule?

Of course! For instance, in hydrogen (H2), the bond length is about 74 picometers, while in ethane (C2H6), the bond lengths of C–C are around 154 picometers. It's fascinating how these values differ, right?

In summary, bond length not only tells us about the distance between atoms but also indicates the bond's strength. Stronger bonds lead to shorter lengths.
Bond Angle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Moving on to our next topic, let’s talk about bond angles. Who can explain what a bond angle is in simple terms?

Isn’t it the angle formed between the bonds of a central atom?

Exactly right! The bond angle is the angle between the orbitals containing bonding electron pairs around a central atom. This measurement helps us define the geometry of molecules. Can anyone give me an example of a molecule and its bond angle?

The bond angle in water (H2O) is less than 109 degrees because it has two lone pairs.

Good job! Water has a bent shape with a bond angle of about 104.5 degrees. The presence of lone pairs affects the angles because they repel more strongly than bond pairs. A simple memory aid to recall this is 'Lone Pairs Push'.

Can you explain how bond angles are measured?

Bond angles can be experimentally determined using techniques like spectroscopy. They provide insights into molecular shapes, which relate directly to how molecules interact with each other.

To summarize, bond angles are crucial for understanding the geometry of molecules and are influenced by the presence of nonbonding electron pairs.
Bond Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s look at bond enthalpy. Who remembers what this term refers to?

Is it the energy required to break a bond?

Spot on! Bond enthalpy is defined as the amount of energy needed to break one mole of bonds of a specific type between two atoms in a gaseous state. Why do you think bond enthalpy is important in chemistry?

It shows how stable or strong a bond is, right?

Exactly! Higher bond enthalpy indicates stronger bonds. For example, the bond enthalpy for H-H in hydrogen gas is 435.8 kJ/mol. As a hint, remember 'High Energy, Strong Bonds' next time!

Does this also vary for different types of bonds?

Absolutely! As bond types go from single to double to triple, their bond enthalpy increases. This is due to greater shared electron density and stronger attractive forces.

In conclusion, understanding bond enthalpy allows us to assess molecular stability and strength effectively.
Bond Order
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let’s learn about bond order. Can someone tell me how we define bond order?

I think it’s the number of bonds between two atoms?

You got it! Bond order is the number of bonds between two atoms in a molecule. It can be calculated as the number of bonded electrons minus antibonded electrons divided by two. Why is this concept critical?

It helps us understand how strong or weak a bond is, right?

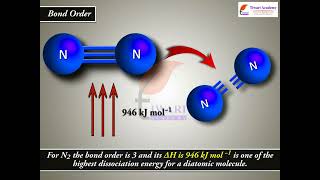
Exactly! A higher bond order correlates with shorter bond lengths and greater strength. For instance, nitrogen (N2) has a bond order of three due to its triple bond, making it very robust.

So, does a single bond have a lower bond order?

Yes! A single bond has a bond order of one, which is weaker compared to multiple bonds. To remember this, think 'More Bonds, More Strength’.

To summarize, bond order provides valuable insights into the strength and characteristics of chemical bonds.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Bond parameters are critical in understanding molecular bonding characteristics. This section outlines various parameters, such as bond length defined by equilibrium distances, bond angles indicative of spatial arrangements, bond enthalpy representing stability through energy measures, bond order correlating to bond strength, and bond polarity affecting interactions among molecules.
Detailed
Bond Parameters
Bond parameters are essential metrics that help in understanding the characteristics of chemical bonds in molecules. This section examines five key parameters:
- Bond Length: Defined as the equilibrium distance between the nuclei of two bonded atoms within a molecule. It's a measure of the size and spatial arrangement of atoms and primarily determined by the type of bond (single, double, or triple). For instance, single bonds have longer bond lengths compared to double or triple bonds.
- Bond Angle: This is the angle between the orbitals containing bonding electron pairs around a central atom in a molecule. It reveals information about the molecule's shape and helps predict the spatial arrangement of atoms.
- Bond Enthalpy: This parameter refers to the amount of energy that is required to break one mole of a particular bond in the gaseous state. Higher bond enthalpy indicates stronger bonds. For example, the H-H bond enthalpy is approximately 435.8 kJ/mol, illustrating its strength.
- Bond Order: This concept refers to the number of bonds between two atoms (e.g., single, double, or triple bonds) and is calculated as the difference between the number of bonding and antibonding electrons divided by two. Therefore, a higher bond order signifies a stronger bond and typically results in shorter bond lengths.
- Polarity of Bonds: No bond is perfectly ionic or covalent; rather, bonds demonstrate varying degrees of character depending upon the electronegativity of the atoms involved. Polar bonds are those in which the shared electrons are drawn closer to one atom due to higher electronegativity, resulting in partial charges.
Understanding these parameters is fundamental for analyzing molecular properties and their behavior in chemical reactions.
Youtube Videos

![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Bond Length
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule. Bond lengths are measured by spectroscopic, X-ray diffraction and electron-diffraction techniques. Each atom of the bonded pair contributes to the bond length. In the case of a covalent bond, the contribution from each atom is called the covalent radius of that atom.
Detailed Explanation
Bond length refers to how far apart the centers of two atoms are from each other when they are covalently bonded. This distance is determined at a point where attractive forces and repulsive forces balance each other, leading to a stable arrangement. The covalent radius is half of the distance between the nuclei of two identical atoms in a molecule. It helps us understand how size affects the bond length; larger atoms will generally have longer bond lengths. Techniques like X-ray diffraction allow scientists to directly measure these distances and understand molecular structures.
Examples & Analogies
Think of two friends with their arms outstretched towards one another. The distance between their fingertips represents the bond length between two atoms. If both friends are tall, their arms would be longer, resulting in a longer distance. Likewise, larger atoms will result in longer bond lengths compared to smaller ones.
Bond Angle
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is defined as the angle between the orbitals containing bonding electron pairs around the central atom in a molecule/complex ion. Bond angle is expressed in degree which can be experimentally determined by spectroscopic methods. It gives some idea regarding the distribution of orbitals around the central atom in a molecule/complex ion and hence it helps us in determining its shape.
Detailed Explanation
The bond angle is crucial for understanding the shape of a molecule. It quantifies the spatial arrangement of the atoms around a central atom, which affects the molecule's overall geometry. For example, in water (H₂O), the angle between the hydrogen atoms is approximately 104.5 degrees, which affects the molecule's 'bent' shape rather than a straight line. This geometry influences physical properties such as boiling and melting points.
Examples & Analogies
Imagine the bond angle as the way you would stretch your arms when posing for a picture with friends. If you spread your arms wide (larger angle), you might be making a circle with your friends. However, if you bring your arms closer together (smaller angle), you create a different, more compact formation. The angle between your arms can represent the bond angle in a molecule.
Bond Enthalpy
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is defined as the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state. The unit of bond enthalpy is kJ mol–1. For example, the H–H bond enthalpy in hydrogen molecule is 435.8 kJ mol–1.
Detailed Explanation
Bond enthalpy provides insight into how strong a bond is between two atoms. A high bond enthalpy value indicates a strong bond that requires significant energy to break. For instance, breaking the H–H bond in a hydrogen molecule requires 435.8 kJ of energy. Understanding bond enthalpy is essential for predicting the stability of compounds and reactions they might undergo.
Examples & Analogies
Consider bond enthalpy like the amount of effort it takes to tear two pages from a tightly bound book. If you need to pull harder to separate two pages (high bond enthalpy), it indicates a strong connection between those pages (strong bond). Conversely, if the pages are loose and come apart easily (low bond enthalpy), it shows a weak connection.
Bond Order
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In the Lewis description of covalent bond, bond order is given by the number of bonds between the two atoms in a molecule. The bond order, for example, in H2 (with a single shared electron pair) is 1, in O2 (with two shared electron pairs) is 2, and in N2 (with three shared electron pairs) is 3.
Detailed Explanation
The concept of bond order helps us assess the strength and stability of a bond in a molecule. Higher bond orders typically correlate with stronger bonds; for instance, triple bonds (like in N2, which has bond order 3) are much stronger and shorter than single bonds (like in H2, which has bond order 1). Bond order can be calculated using the formula which depends on the electrons present in bonding and antibonding orbitals.
Examples & Analogies
Think of bond order like different layers of security on a door. A single lock (single bond) keeps the door locked, but it's easier to break in compared to a double lock (double bond). Now, consider a reinforced door with multiple locks (triple bond) – it's much harder to break in due to the higher level of security.
Resonance Structures
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is often observed that a single Lewis structure is inadequate for the representation of a molecule in conformity with its experimentally determined parameters. For example, the ozone, O3 molecule can be equally represented by the structures I and II shown. In both structures we have a O–O single bond and a O=O double bond. The normal O–O and O=O bond lengths are 148 pm and 121 pm respectively.
Detailed Explanation
Resonance structures allow for a more accurate depiction of the actual bonding in molecules where one Lewis structure cannot fully represent all aspects of bonding. For example, ozone (O3) does not accurately depict the bond properties with a single structure. Instead, it can be portrayed as an average of two or more structures that contribute to its actual state, resulting in bond lengths that are intermediate between those of a single and double bond.
Examples & Analogies
Consider resonance like different ways to describe a chameleon that changes color based on its surroundings. While one description might focus on it being green (representing one Lewis structure), it might also be brown or yellow depending on the background. Just like a single description is inadequate, multiple resonance structures help provide a fuller picture of the molecule's characteristics.
Key Concepts
-
Bond Length: Measures the distance between bonded atoms, crucial for determining bond type.
-
Bond Angle: Indicates the spatial arrangement of atoms and affects molecular geometry.
-
Bond Enthalpy: Energy needed to break a bond, signifying bond strength.
-
Bond Order: The relationship between bond strength and bond length; higher order means shorter and stronger bonds.
-
Polarity: The distribution of charge across bonds, influencing molecular interactions.
Examples & Applications
Example 1: The bond length in a hydrogen molecule (H2) is about 74 picometers.
Example 2: A water molecule (H2O) has a bond angle of 104.5 degrees.
Example 3: The bond enthalpy for H-H in hydrogen is 435.8 kJ/mol, indicating a strong single bond.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Bond length shows how far apart they stay, while bond angle keeps their shapes at bay.
Stories
Imagine two hydrogen atoms that dance together at a specific distance, forming a strong bond and an angle that keeps them united, showing us strength and stability.
Memory Tools
Use 'LEAP' for remembering bond properties: Length, Angle, Enthalpy, Polarity.
Acronyms
B.E.A.S.P for Bond Enthalpy, Angle, Strength, and Polarity.
Flash Cards
Glossary
- Bond Length
The equilibrium distance between the nuclei of two bonded atoms.
- Bond Angle
The angle formed between the orbitals containing bonding pairs of electrons around a central atom.
- Bond Enthalpy
The amount of energy required to break one mole of bonds in a gaseous state.
- Bond Order
The number of bonds between two atoms, calculated as (number of bonding electrons - number of antibonding electrons)/2.
- Polarity
The degree to which a bond or molecule has positive and negative charges due to electronegativity differences between bonding atoms.
Reference links
Supplementary resources to enhance your learning experience.
