HYBRIDISATION
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Hybridization
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're going to discuss hybridization, which is a process crucial in understanding the shapes of molecules. Can anyone tell me what they think hybridization involves?

Is it about how orbitals mix together?

Exactly, Student_1! Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals. These new orbitals can make stronger bonds. Now, what are some typical geometries we need to consider?

I think linear and trigonal planar are some of them?

Good memory! We have linear geometry for sp hybridization and trigonal planar for sp2. And what about tetrahedral geometry?

That's for sp3 hybridization, like in methane!

Well done! Remembering that methane uses sp3 hybridization is important. Let's wrap up this session: hybridization helps explain the shapes and bond angles in many molecules.
Types of Hybridization
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In our last session, we touched on hybridization. Let’s discuss its types in detail. Who can explain sp hybridization?

Sp hybridization mixes one s and one p orbital, resulting in two sp orbitals, which have linear geometry.

Perfect! And what about sp2 hybridization?

That one involves one s and two p orbitals forming three sp2 orbitals with trigonal planar geometry.

Great job! And sp3?

It combines one s and three p orbitals creating four sp3 orbitals that are tetrahedral.

Exactly! Remember the 109.5-degree bond angles in sp3. These concepts of hybridization are essential when predicting molecular shapes!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Hybridization is introduced as a significant concept to explain the geometric arrangements of atoms in polyatomic molecules. It encompasses the mixture of atomic orbitals to form hybrid orbitals, allowing the understanding of molecular bonding and structure, particularly in molecule types such as sp, sp2, and sp3 hybridizations.
Detailed
Detailed Summary
Hybridization is the process of combining atomic orbitals of differing energies to create new hybrid orbitals that are equivalent in shape and energy. This concept was introduced by Linus Pauling to explain the geometrical structures observed in several polyatomic molecules. Each hybrid orbital formed is directed in a way that reduces repulsions, leading to lower energy configurations.
Key Points Covered:
- Definition & Importance of Hybridization: Hybridization describes how orbitals mix to form new hybrid orbitals, which are more effective in bonding than the original atomic orbitals. This is crucial in understanding molecular geometry.
- Types of Hybridization: The section covers three primary types:
- sp Hybridization: Involves one s and one p orbital mixing to form two equivalent sp orbitals with linear geometry.
- sp2 Hybridization: Involves one s and two p orbitals forming three equivalent sp2 orbitals with trigonal planar geometry.
- sp3 Hybridization: Involves one s and three p orbitals forming four equivalent sp3 orbitals with tetrahedral geometry.
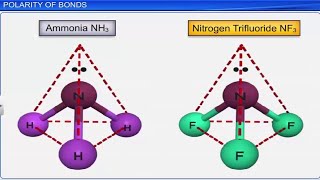
- Application of Hybridization: Understanding hybridization helps in predicting the molecular structures of compounds like CH4 (methane), NH3 (ammonia), and H2O (water), explaining their bond angles and shapes based on the number of hybrid orbitals formed.
- Hybridization of d Orbitals: The section also discusses hybridization involving d orbitals, significant in elements from the third period and beyond, leading to complex geometries such as those in PCl5 and SF6.
Understanding hybridization is critical for comprehending molecular shapes and bonding behaviors, thus linking atomic structure with chemical properties.
Youtube Videos

![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)





Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Hybridisation
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In order to explain the characteristic geometrical shapes of polyatomic molecules like CH4, NH3 and H2O etc., Pauling introduced the concept of hybridisation. According to him the atomic orbitals combine to form new set of equivalent orbitals known as hybrid orbitals.
Detailed Explanation
Hybridisation is a concept that helps explain the shapes and bonding of certain molecules. When atomic orbitals combine, they form new orbitals called hybrid orbitals that are equivalent in energy. For example, when carbon forms methane (CH4), its orbitals mix to create four equivalent hybrid orbitals that point toward the corners of a tetrahedron, giving the molecule its distinctive shape.
Examples & Analogies
Imagine mixing paint colors. When you combine blue and yellow paint, you get green, which has properties different from the original colors. Similarly, atomic orbitals mix to form hybrid orbitals that lead to new shapes, just like mixing paints gives you a new color.
Salient Features of Hybridisation
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The main features of hybridisation are as under:
1. The number of hybrid orbitals is equal to the number of the atomic orbitals that get hybridised.
2. The hybridised orbitals are always equivalent in energy and shape.
3. The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
4. These hybrid orbitals are directed in space in some preferred direction to have minimum repulsion between electron pairs and thus a stable arrangement.
Detailed Explanation
Key features of hybridisation include: 1) the count of hybrid orbitals matches the atomic orbitals involved; 2) all hybrid orbitals have the same energy and shape, making them equivalent; 3) these hybrid orbitals form stronger bonds compared to pure atomic orbitals; and 4) the spatial arrangement of hybrid orbitals minimizes electron repulsion, resulting in a stable molecular structure.
Examples & Analogies
Think of hybridisation like a group of friends rearranging themselves for a photo. Just as they position themselves to maximize space and minimize crowding, hybrid orbitals arrange themselves to minimize repulsion, ensuring the best possible structure for the molecule.
Types of Hybridisation
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
There are various types of hybridisation involving s, p and d orbitals. The different types of hybridisation are as under:
(i) sp hybridisation: This type of hybridisation involves the mixing of one s and one p orbital resulting in the formation of two equivalent sp hybrid orbitals.
(ii) sp2 hybridisation: In this hybridisation, there is involvement of one s and two p-orbitals in order to form three equivalent sp2 hybridised orbitals.
(iii) sp3 hybridisation: This type of hybridisation can be explained by taking the example of CH4 molecule, where mixing occurs to form four sp3 hybrid orbitals.
Detailed Explanation
Hybridisation can be classified into types based on the orbitals mixed. For example, in sp hybridisation, one s orbital and one p orbital mix to produce two linearly arranged sp orbitals. In sp2 hybridisation, one s and two p orbitals create three planar sp2 orbitals, as seen in molecules like BCl3. In sp3 hybridisation, one s and three p orbitals combine to form four sp3 orbitals directed towards the corners of a tetrahedron, which is what occurs in methane (CH4).
Examples & Analogies
Imagine creating different types of clay shapes. If you mix two colors to create a line shape, that's like sp hybridisation. If you spread your clay to form a triangle, that's like sp2. Finally, if you mold it into a pyramid, that's sp3, just like methane’s shape. Each type of hybridisation tells us how atoms bond differently, just as different shapes can tell us how to use the clay.
Key Concepts
-
Hybridization: Mixing of atomic orbitals to form hybrid orbitals.
-
sp Hybridization: Linear geometry formed from one s and one p orbital.
-
sp2 Hybridization: Trigonal planar geometry formed from one s and two p orbitals.
-
sp3 Hybridization: Tetrahedral geometry formed from one s and three p orbitals.
Examples & Applications
The sp hybridization in BeCl2 gives it a linear geometry.
In CH4, sp3 hybridization results in a tetrahedral shape.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In BeCl2, long and straight, sp hybrids make it great.
Stories
Imagine a party where the sp3 hybridized carbon brings four friends, spreading them out in a tetrahedral dance.
Memory Tools
Hybridization Types: 'Spicy Spaghetti Stir-fry'—sp, sp2, and sp3.
Acronyms
Remember 'SHADE' for sp Hybridization (S for Straight, H for Hybrid, A for Angled, D for Directional, E for Energies equal).
Flash Cards
Glossary
- Hybridization
The process of intermixing atomic orbitals to form new hybrid orbitals with equivalent energies and shapes.
- sp Hybridization
A form of hybridization involving the mixing of one s and one p orbital to form two equivalent sp orbitals.
- sp2 Hybridization
A form involving one s and two p orbitals, resulting in three equivalent sp2 orbitals with trigonal planar geometry.
- sp3 Hybridization
A hybridization that combines one s and three p orbitals to create four sp3 orbitals with tetrahedral geometry.
- Bond Angle
The angle formed between two covalent bonds that originate from the same atom, indicating molecular shape.
Reference links
Supplementary resources to enhance your learning experience.
