Electronic Configuration and Molecular Behaviour
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Molecular Orbitals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we'll discuss molecular orbitals, which are key in understanding a molecule's behavior. Can anyone tell me what happens to atomic orbitals when they combine to form molecular orbitals?

They mix to form new orbitals that belong to the entire molecule, right?

Exactly! When atomic orbitals combine, they create bonding and antibonding molecular orbitals. Who can tell me the significance of these classifications?

Bonding molecular orbitals stabilize the molecule, while antibonding orbitals can destabilize it.

Correct! Remember: more electrons in bonding orbitals lead to greater stability. What is the bond order?

Bond order is half the difference between bonding and antibonding electrons!

Good job! Always remember that a higher bond order indicates a stronger bond. Let’s recap: bonding orbitals stabilize, antibonding orbitals destabilize, and bond order helps us understand stability.
Stability of Molecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve deeper into the stability of molecules. What determines if a molecule is stable or unstable?

It’s based on the comparison between Nb and Na, right?

Exactly! If Nb is greater than Na, the molecule is stable. Can anyone give me an example of a stable molecule?

Oxygen, O2, is an example since it has a bond order of 2.

Absolutely! And how about an unstable example?

He2 does not exist because its bond order is zero!

Well done! The bond order concept is fundamental. Let’s summarize: stability relates to Nb and Na, with higher bonding distributions indicating stability.
Magnetic Properties of Molecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will also explore magnetic properties. Who can explain how magnetic behavior is determined by electrons in molecular orbitals?

If all electrons in the orbitals are paired, the molecule is diamagnetic. If there are unpaired electrons, it's paramagnetic.

Correct! Can anyone provide a real-life example of a paramagnetic molecule?

O2 is paramagnetic because it has unpaired electrons in its molecular orbitals!

Excellent example! Remember, the presence of unpaired electrons is key to magnetic properties. In summary: diamagnetic means all pairs are filled, while paramagnetic has unpaired electrons.
Understanding Bond Order and Stability
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s revisit bond order—it’s essential for understanding molecular stability. Can someone recap what bond order means?

Bond order is calculated as ½(Nb – Na), right?

Right! And what does a positive bond order indicate?

A stable molecule!

Correct again! And what about a zero bond order?

It indicates instability or that the molecule doesn’t exist.

Exactly! Let's summarize today’s discussion: bond order relates directly to stability, with positive values indicating stability and zero values indicating instability.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
It discusses how the distribution of electrons among molecular orbitals informs the stability, bond order, and magnetic properties of molecules. The section highlights the criteria for stable molecules and the nature of bonds formed based on their electronic configurations.
Detailed
Electronic Configuration and Molecular Behaviour
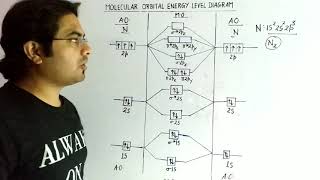
The distribution of electrons among various molecular orbitals plays a crucial role in determining the stability and properties of molecules. In molecular orbital theory, molecular orbitals are formed via the combination of atomic orbitals, which leads to the formation of bonding and antibonding orbitals. Stability is defined based on the occupancy of these orbitals; a molecule is stable when the number of electrons in bonding orbitals (Nb) exceeds those in antibonding orbitals (Na).
Key Points:
- Bond Order: Bond order (b.o.) is calculated as ½(Nb – Na). Higher bond orders indicate more stable bonds.
- Magnetic Properties: Molecules are classified as diamagnetic if all orbital electrons are paired and paramagnetic if there are unpaired electrons.
- Understanding molecular behavior involves analyzing electronic configurations, which provide insights into bond strength, length, and overall molecular stability.
Therefore, this section emphasizes the application of electronic configuration in predicting molecular characteristics and stability, integrating concepts of bonding interactions between atoms.
Youtube Videos

![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Stability of Molecules
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If Nb is the number of electrons occupying bonding orbitals and Na the number occupying the antibonding orbitals, then
(i) the molecule is stable if Nb is greater than Na, and
(ii) the molecule is unstable if Nb is less than Na.
Detailed Explanation
In chemical bonds, the occupancy of electrons in the molecular orbitals dictates the overall stability of a molecule. When there are more electrons in the bonding orbitals than in the antibonding orbitals, the molecule gains stability. Conversely, if the electrons start filling the antibonding orbitals, it results in instability. Thus, the balance between bonding and antibonding electrons is crucial in determining whether a molecule will exist in a stable form or not.
Examples & Analogies
Think of it like a team sport. If the players (electrons) on your team (bonding orbitals) outnumber those on the opposing team (antibonding orbitals), your team is likely to win (the molecule is stable). However, if the opposing team starts to have more players, your team's chances of winning decline (the molecule becomes unstable).
Bond Order Concept
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
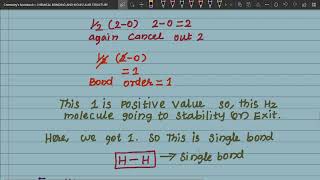
Bond order (b.o.) is defined as one half the difference between the number of electrons present in the bonding and the antibonding orbitals i.e., Bond order (b.o.) = ½ (Nb – Na).
Detailed Explanation
Bond order is a quantifiable way to assess the strength and stability of a bond between two atoms. A positive bond order indicates that there are more bonding electrons (which help hold the atoms together) than antibonding electrons (which work against bond formation). For instance, a bond order of 1 implies a single bond, 2 indicates a double bond, and 3 signifies a triple bond. The higher the bond order, the stronger the bond, which is linked to the shorter bond length as well.
Examples & Analogies
Imagine bond order like the health of a bridge. A strong bridge (high bond order) can support more traffic (stronger bond), but if cracks form (more antibonding electrons), it can become weaker and fracture under less weight (lower stability). Just like a bridge's safety rating, bond order gives insight into how well atoms can stick together.
Impact of Bond Order on Stability and Properties
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The rules discussed regarding the stability of the molecule can be restated in terms of bond order as follows: A positive bond order (i.e., Nb > Na) means a stable molecule while a negative (i.e., Nb
Detailed Explanation
This section emphasizes that bond order not only reflects stability but also gives insights into the nature of the bond. For instance, if the bond order is greater than zero, the bond is likely to exist in a stable state. If we encounter a negative or zero bond order, it indicates that the molecule may not be stable and is likely to break apart. This concept directly correlates to the energetic balance in molecular interactions.
Examples & Analogies
Consider bond order like the fuel of a vehicle. A car with full fuel (positive bond order) can drive smoothly, indicating stability. If the fuel tank is empty or has leaks (zero or negative bond order), the vehicle is bound to stop functioning. Therefore, just like checking a car's fuel gauge before a trip, bond order offers a clear indication of the 'energy reserve' and stability of a molecule.
Bond Length and Bond Order Relationship
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The bond length decreases as bond order increases.
Detailed Explanation
Bond length is inversely related to bond order. This means that as we form more bonds between two atoms (single, double, triple), the distance between them (bond length) decreases. Essentially, more shared electrons pull the atoms closer together, increasing the bond strength and shortening the distance. This relationship is crucial for understanding how atoms interact in compounds.
Examples & Analogies
Imagine holding hands with someone. If you’re just holding hands lightly (single bond), there’s a fair distance between you two (longer bond length). However, if you both pull in closer and hug (double or triple bond), that distance decreases significantly! Just like in chemistry, holding on tighter (more bonds) makes you feel closer.
Magnetic Properties of Molecules
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If all the molecular orbitals in a molecule are doubly occupied, the substance is diamagnetic (repelled by magnetic field). However if one or more molecular orbitals are singly occupied, it is paramagnetic (attracted by magnetic field), e.g., O2 molecule.
Detailed Explanation
Magnetic properties of molecules flow from the arrangement of their electrons in molecular orbitals. In a diamagnetic substance, all electrons are paired, resulting in no net magnetic moment; these materials are not attracted to magnetic fields. On the other hand, paramagnetic materials possess unpaired electrons, leading to a net magnetic moment; they are attracted to external magnetic fields. For example, oxygen molecules (O2) are paramagnetic due to their two unpaired electrons in the π* molecular orbitals.
Examples & Analogies
Think of molecules as party attendees. In a 'party' with all pairs dancing together (like in diamagnetics), there’s no one single left out (no unpaired electrons). But if some attendees choose to dance alone (like in paramagnetics), they create excitement and draw attention, becoming the life of the party (attraction by a magnetic field).
Key Concepts
-
Bond Order: A value indicating the strength and stability of a bond.
-
Molecular Orbitals: Regions of space where electrons reside in a molecule.
-
Stability: Determined by comparing the number of electrons in bonding vs. antibonding orbitals.
-
Magnetic Properties: Depend on the presence of unpaired or paired electrons in molecular orbitals.
Examples & Applications
Oxygen molecule (O2) with bond order of 2 is stable and paramagnetic due to unpaired electrons.
Helium molecule (He2) has bond order of 0, thus, it is unstable and does not exist.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Bond order is the key, nb over na, it's the safety.
Stories
Imagine two friends deciding who gets to ride the front seat in a car. If one of them has more toys to share, they get to lead. This mirrors bond order—more in bonding means a stable ride!
Memory Tools
B.O.N.D: Bond Order = Nb – Na; Over Stability by determining if it’s stable or not; Natural behavior shows if it's magnetic.
Acronyms
BOND
Bond Order = Number of electrons in bonding – Number of electrons in antibonding divided by 2.
Flash Cards
Glossary
- Molecular Orbitals
Regions in a molecule where electrons are likely to be found, formed from the combination of atomic orbitals.
- Bonding Molecular Orbital
A molecular orbital that results from the constructive overlap of atomic orbitals, stabilizing the molecule.
- Antibonding Molecular Orbital
A molecular orbital formed by the destructive interference of atomic orbitals, destabilizing the molecule.
- Bond Order
A measure of the stability of a bond, calculated as ½(Nb – Na), where Nb is the number of electrons in bonding orbitals and Na is the number in antibonding orbitals.
- Diamagnetic
A property of a substance with all electrons paired, causing it to be weakly repelled by a magnetic field.
- Paramagnetic
A property of a substance with unpaired electrons, causing it to be attracted by a magnetic field.
Reference links
Supplementary resources to enhance your learning experience.
