Octet Rule
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the Octet Rule
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss a fundamental concept in chemistry known as the Octet Rule. Can anyone tell me what the Octet Rule states?

Is it about how atoms want to have eight electrons?

Exactly! The Octet Rule suggests that atoms will gain, lose, or share electrons to achieve a stable configuration of eight electrons in their outer shell, similar to that of noble gases.

Why is having eight electrons so important?

Great question! Atoms with eight electrons in their valence shells are generally more stable and less reactive. This configuration is energetically favorable, allowing them to form strong bonds with other atoms.

What about atoms with fewer than eight electrons?

Atoms with fewer than eight electrons will often form bonds with other atoms to reach that stable state. For instance, sodium donates an electron to chlorine to form NaCl, achieving octets for both.

Can you give us an example of how it works with covalent bonds?

Sure! In the case of water, H2O, each hydrogen atom shares one electron with the oxygen atom, allowing all atoms involved to complete their octets.

To summarize, the Octet Rule helps us understand how atoms interact to achieve stability. We'll dive deeper into its applications soon!
Ionic vs. Covalent Bonds
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've grasped the Octet Rule, let's differentiate between ionic and covalent bonds. Can anyone explain how these two types of bonding relate to the Octet Rule?

I think ionic bonds form when atoms transfer electrons, right?

That's correct! In ionic bonding, one atom donates electrons to achieve a full octet, while the other atom receives them. For example, sodium (Na) transfers an electron to chlorine (Cl) to form Na+ and Cl- ions.

And what about covalent bonds?

Covalent bonding involves sharing electrons. For instance, in a molecule like CO2, carbon shares electrons with two oxygen atoms, achieving an octet for all involved.

Are there exceptions to the Octet Rule?

Yes, there are exceptions like incomplete octets in some elements like beryllium or odd-electron molecules like nitrogen dioxide. We'll explore those exceptions more in our next session.

In summary, understanding ionic and covalent bonds through the lens of the Octet Rule allows us to predict how elements will interact to achieve stability.
Limitations of the Octet Rule
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

While the Octet Rule is a valuable guideline, it does have limitations. Can anyone name one limitation?

Maybe elements like beryllium don't follow the rule?

Exactly! Beryllium often has only four electrons around it in compounds. Other exceptions include molecules with odd numbers of electrons, such as NO2.

What if there are elements that can have more than eight electrons?

Excellent point! Some elements can expand their octets, particularly those in the third period or beyond, like phosphorus in PCl5. They can utilize d orbitals to accommodate more than eight electrons.

So how does this affect our understanding of chemical bonding?

It means that while the Octet Rule helps us make predictions, we also need to recognize when it's not applicable. Understanding these limitations aids in a more comprehensive grasp of chemical structure.

To summarize today, we learned about the limitations of the Octet Rule, such as exceptions and the expanded octet concept, enhancing our understanding of chemical bonding.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The Octet Rule is a critical theory in chemistry that describes how atoms combine to achieve a stable electronic configuration resembling that of noble gases. This rule is foundational in understanding ionic and covalent bonding.
Detailed
Octet Rule
The Octet Rule is a principle in chemistry that states that atoms tend to bond in such a way that they each have eight electrons in their valence shells. This rule helps explain the chemical bonding and molecular structure of many compounds. Developed by Kössel and Lewis in 1916, it emphasizes that by gaining, losing, or sharing electrons, atoms can attain an octet configuration, which leads to increased stability similar to that of noble gases. While the Octet Rule provides a useful framework for understanding bonding, there are exceptions where the rule does not apply, such as incomplete octets, odd-electron molecules, and expanded octets involving elements from the third period and beyond. Understanding the Octet Rule allows for better predictions in chemical reactivity and molecular geometry.
Youtube Videos


![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to the Octet Rule
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
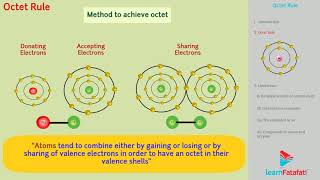
Kössel and Lewis in 1916 developed an important theory of chemical combination between atoms known as electronic theory of chemical bonding. According to this, atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an octet in their valence shells. This is known as octet rule.
Detailed Explanation
The octet rule is a fundamental concept in chemistry that explains how atoms bond with each other. In simple terms, it states that atoms tend to form bonds in such a way that they each end up with eight electrons in their outermost shell, similar to the electron configuration of noble gases which are inherently stable. This can happen through transferring electrons (like in ionic bonds) or sharing electrons (like in covalent bonds).
Examples & Analogies
Imagine each atom as a soccer player trying to form a team of eight players. The octet rule functions like a rule stating that a successful team needs at least eight players. Some players achieve this by passing the ball (electrons share) among themselves to reach eight, while others may have to 'drop' (lose) some players (electrons) to others to keep their team compliant with the 'eight-player' requirement.
Implications of the Octet Rule
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The octet rule is particularly crucial for understanding the stability of atoms and predicting the types of bonds they will form. It implies that most elements will react in ways that allow them to attain a noble gas electron configuration, leading to a more stable state.
Detailed Explanation
Due to the octet rule, many elements in the periodic table strive to achieve this state of stability. This explains why atoms tend to form bonds: by either giving away, accepting, or sharing electrons, they can transform from unstable single atoms into stable molecules. It helps predict how elements will behave during reactions and how they form compounds, guiding chemists in their studies of material properties.
Examples & Analogies
Think of it like a group project in school where each group member wants to finish the project effectively. To do this, they may give some resources to others or share their materials. Similarly, in a chemical reaction, atoms will bond in ways that allow them to each reach their 'eight electron' goal, just like each group member aims for the best outcome in the project.
Examples of the Octet Rule in Action
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A common example illustrating the octet rule is the formation of sodium chloride (NaCl). Sodium (Na) donates an electron to chlorine (Cl), leading sodium to attain a stable configuration akin to neon. Chlorine, receiving that electron, reaches a stable configuration as well, resembling argon.
Detailed Explanation
In this case, sodium starts with one electron in its outer shell and wants to lose it to be stable, while chlorine has seven and needs one more. By giving away the electron, sodium achieves stability with eight electrons in its inner shell, and chlorine becomes stable by filling its outer shell to eight. This transfer results in the formation of Na+ and Cl- ions, which then attract each other due to opposite charges, forming NaCl.
Examples & Analogies
Imagine sodium as a kid with a toy who wants to play with more toys. He gives away one toy (the electron) to another kid (chlorine), who needs that toy to feel complete in their play. Once this sharing happens, both kids are happier (more stable), and they decide to stick together as friends (ionic bond) because they exchanged something valuable!
Key Concepts
-
Octet Rule: Atoms tend to bond to achieve eight electrons in their valence shell.
-
Covalent Bond: Atoms share electrons to complete octets.
-
Ionic Bond: One atom transfers electrons to another to achieve stability.
-
Lewis Dot Structures: Visual representation showing the bonding in a molecule.
Examples & Applications
Sodium and chlorine form NaCl through the transfer of an electron from sodium to chlorine.
In water (H2O), hydrogen atoms share electrons with oxygen to complete their octets.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In the Octet, stability we seek, eight electrons make atoms less weak.
Stories
Imagine atoms at a party; they want to pair up (form bonds) to feel complete, just like in life!
Memory Tools
Noble Gases (He, Ne, Ar, Kr, Xe) have full 'octets' because they're best positioned, no need for more electrons.
Acronyms
BONDS (Binds with Octets, Noble gas configuration Decreases reactivity Stability).
Flash Cards
Glossary
- Octet Rule
A chemical rule that reflects the tendency of atoms to prefer to have eight electrons in their valence shell.
- Covalent Bond
A type of chemical bond where atoms share electron pairs.
- Ionic Bond
A chemical bond formed between two ions with opposite charges, typically involving the transfer of electrons.
- Valence Electrons
Electrons in the outer shell of an atom that are involved in forming bonds.
- Lewis Dot Structure
A diagram that shows the bonding between atoms of a molecule and the lone pairs of electrons.
- Electronegativity
A measure of the tendency of an atom to attract a bonding pair of electrons.
- Expanded Octet
A situation where an atom has more than eight electrons in its valence shell.
Reference links
Supplementary resources to enhance your learning experience.
