Lewis Representation of Simple Molecules (the Lewis Structures)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Lewis Structures
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome, class! Today, we'll explore Lewis structures, which are essential for understanding how atoms bond in molecules. Can anyone tell me what a Lewis structure represents?

It shows how atoms are connected using valence electrons, right?

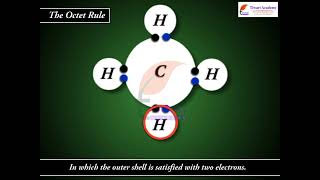
Exactly! Lewis structures use dots to represent valence electrons and lines for bonds. Remember: every atom aims for an octet.

What is an octet?

Good question! The octet rule states that atoms tend to bond in ways that give them eight valence electrons. Let's keep that in mind.
Steps to Draw Lewis Structures
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we have a basic concept, let's go through the steps to draw Lewis structures. Who can recall the first step?

We need to count the total valence electrons, right?

Great! And why do we do that?

To know how many electrons we can use to make bonds!

Correct! Let's say we're drawing the structure for CO2. Can anyone share how many valence electrons we need?

Carbon has 4, and each oxygen has 6, that makes 16 total!

Exactly! Now, according to our steps, what do we do next?
Connecting Atoms and Filling Octets
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

After counting valence electrons, we connect the atoms. Who can describe how we would connect Carbon and Oxygen in CO2?

We would draw one bond between Carbon and each Oxygen.

Right again! But what do we do if the octets are not complete?

We could form double bonds to share more electrons!

Exactly! CO2 needs double bonds to satisfy the octet rule. Always remember, octet rules help in creating stable structures.
Practice and Application
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's practice! Draw the Lewis structure for H2O. What is the first step?

Count the electrons! Oxygen has 6 and each Hydrogen has 1.

Good. So how many electrons do we have to work with?

That makes 8 total!

Exactly! Make your connections, and don't forget to check your octets. Let's finish up these structures.
Review and Key Takeaways
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we wrap up, what is the main purpose of drawing Lewis structures?

To show how atoms connect and share electrons!

Excellent! This helps us predict molecular shape and properties. Keep practicing and remember: every atom seeks stability.

What if there's an exception to the octet rule?

Great question! Some molecules don't follow this rule, which we'll discuss in future lessons. Until next time!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section outlines the steps required to draw Lewis structures, emphasizing the importance of counting valence electrons, arranging atoms in a skeletal structure, and making sure each atom adheres to the octet rule. Examples and steps for Lewis structures of various molecules are provided to enhance understanding.
Detailed
Lewis Representation of Simple Molecules
The Lewis structures provide a visual representation of how atoms in a molecule are bonded using shared pairs of electrons and their lone pairs, facilitating a better understanding of molecular formation and properties.
Key Points:
- Valence Electrons Counting: Begin by calculating the total number of valence electrons from the atoms involved in the molecule. For example, in methane (CH4), there are 8 valence electrons total (4 from Carbon and 4 from Hydrogen).
- Arrangement of Atoms: Determine the skeletal structure before proceeding to share electron pairs. The least electronegative atom usually occupies the center.
- Bond Formation: A single covalent bond is depicted by a pair of shared electrons (dots), however, covalent bonds can also involve multiple pairs.
- Augmenting Octets: After forming single bonds, make adjustments to ensure that each atom reaches an octet of electrons, if possible.
Importance of Lewis Structures:
Lewis structures are essential for predicting the arrangement of electrons in molecules, providing key insights into molecular geometry and polarity.
Youtube Videos




![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Lewis Dot Structures
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule. While such a picture may not explain the bonding and behaviour of a molecule completely, it does help in understanding the formation and properties of a molecule to a large extent. Writing of Lewis dot structures of molecules is, therefore, very useful.
Detailed Explanation
Lewis dot structures are visual representations that show how atoms in a molecule are bonded through shared pairs of electrons. The concept is grounded in the octet rule, which dictates that atoms tend to form bonds until they are surrounded by eight electrons, leading to stability. This representation may simplify complex molecular behaviors but is vital for grasping how molecules interact.
Examples & Analogies
Think of water as a molecule where the oxygen atom shares its electrons with hydrogen atoms. In a Lewis structure of water (H2O), we show the oxygen in the center with two pairs of dots representing bonds to two hydrogen atoms, illustrating how the atoms come together to form a stable entity, much like how members of a community interact and support each other.
Steps to Write Lewis Dot Structures
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The Lewis dot structures can be written by adopting the following steps:
- The total number of electrons required for writing the structures are obtained by adding the valence electrons of the combining atoms. For example, in the CH4 molecule there are eight valence electrons available for bonding (4 from carbon and 4 from the four hydrogen atoms).
- For anions, each negative charge would mean addition of one electron. For cations, each positive charge would result in subtraction of one electron from the total number of valence electrons. For example, for the CO32– ion, the two negative charges indicate that there are two additional electrons than those provided by the neutral atoms. For NH4+ ion, one positive charge indicates the loss of one electron from the group of neutral atoms.
- Knowing the chemical symbols of the combining atoms and having knowledge of the skeletal structure of the compound, it is easy to distribute the total number of electrons as bonding shared pairs between the atoms in proportion to the total bonds.
Detailed Explanation
To properly write a Lewis dot structure, follow these steps: First, determine the total number of valence electrons by adding the electrons from each atom involved, accounting for any charges. For example, methane (CH4) requires 8 electrons—4 from carbon and 4 from the four hydrogens. Next, catalog changes from charges; negatively charged ions gain electrons while positively charged ions lose electrons. Finally, arrange the atoms in a skeletal structure and distribute the electrons as bonds, ensuring every atom achieves stability via an octet.
Examples & Analogies
Imagine creating a seating arrangement for a dinner party. Start by counting how many guests (electrons) you can accommodate based on the number of chairs (valence electrons). If someone brings a friend (an anion), you add more chairs. If someone cancels (a cation), you remove a chair. After determining the total, arrange your guests around the table (molecule) ensuring no one is left standing (all atoms fill their bonding needs).
Determining Central Atoms and Bonding
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In general, the least electronegative atom occupies the central position in the molecule/ion. For example, in the NF3 and CO32–, nitrogen and carbon are the central atoms whereas fluorine and oxygen occupy the terminal positions. After accounting for the shared pairs of electrons for single bonds, the remaining electron pairs are either utilized for multiple bonding or remain as lone pairs.
Detailed Explanation
When forming a Lewis structure, the least electronegative atom is often chosen as the central atom. This central atom forms bonds with surrounding atoms, which are more electronegative. In NF3, nitrogen is the central atom connected to three fluorine atoms. After determining how many bonds form based on shared electrons, any leftover electrons can either participate in additional bonding as lone pairs or remain unshared.
Examples & Analogies
Consider a music band where one musician (the central atom) plays a solo while the others (surrounding atoms) harmonize. In a song, the guitar might take the lead, surrounded by vocalists. The coordination between parts leads to a balanced performance (the stable structure). Just like lingering notes can subtly support the music without overwhelming it, lone pairs make the molecule stable without forming additional bonds.
Examples of Lewis Structures
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Lewis representations of a few molecules/ions are given in Table 4.1.
Detailed Explanation
Table 4.1 showcases examples of common molecules and their respective Lewis structures, illustrating how atoms bond together through shared electron pairs. These structures visually represent electron pair distribution, providing insight into molecular behavior and stability.
Examples & Analogies
Think of these structures as blueprints for building a house. Each house (molecule) has a specific plan (Lewis structure) that details where each room (atom) goes and how they connect (bonds vary). Just as a builder follows a blueprint, chemists use these representations to understand how molecules form and interact in nature.
Practical Problem-Solving with Lewis Structures
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let's solve a couple of example problems:
Problem 4.1: Write the Lewis dot structure of the CO molecule.
- Step 1: Count the total number of valence electrons from carbon (4) and oxygen (6), giving us 10.
- Step 2: Write the skeletal structure: C - O.
- Step 3: Draw a single bond (one shared electron pair) between C and O, completing the octet on oxygen and adjusting for carbon via multiple bonding.
Problem 4.2: Write the Lewis structure of the nitrite ion, NO2–.
- Step 1: Count valence electrons for nitrogen (5) and two oxygen atoms (6 each), plus one for the charge, resulting in 18.
- Step 2: Skeletal structure: O - N - O.
- Step 3: Draw the bonds adequately meeting the octet rule and adjust for double bonding if necessary.
Detailed Explanation
Practical examples help solidify the understanding of Lewis structures. For CO, the process involves counting valence electrons, setting up a skeletal structure, then determining bonds between atoms. For the nitrite ion, you follow similar steps, ensuring to adjust for the overall charge and correctly represent bonding.
Examples & Analogies
Think about these problems as recipes for cooking. Each ingredient (electron) needs to be measured carefully to get the perfect dish (molecule). When writing a Lewis structure, you follow the steps like a recipe—check ingredients, arrange them accordingly, and improvise if necessary—like adjusting spices (bonds) to ensure the final dish is satisfying (stable).
Key Concepts
-
Lewis Structure: A representation of a molecule's bonding and electron arrangement.
-
Valence Electrons: Electrons available for bonding in an atom.
-
Covalent Bond: A bond formed by the sharing of electron pairs between atoms.
-
Octet Rule: Atoms bond to achieve a full outer shell of eight electrons.
Examples & Applications
Example of CO2: To draw its Lewis structure, start with 16 valence electrons, connect carbon and oxygen with double bonds.
Example of H2O: Count 8 valence electrons, connect oxygen to two hydrogens, ensuring octets are complete.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Lewis dots mean a chemical plot, sharing electrons to hit the spot.
Stories
In a kingdom of atoms, each wanted to fit in. They shared their electrons in bonds and began a new kin!
Memory Tools
D-O-C: Dots signify Electrons, Octet Rule, Connect Atoms.
Acronyms
LEWIS
Lewis Electron Wards Include Sharing.
Flash Cards
Glossary
- Lewis Structure
A diagram that represents the bonds between atoms in a molecule using dots to indicate valence electrons.
- Valence Electrons
Electrons in the outermost shell of an atom, which are involved in forming bonds.
- Octet Rule
A principle stating that atoms tend to bond until they have eight electrons in their valence shells.
- Covalent Bond
A type of chemical bond where two atoms share one or more pairs of electrons.
- Polar Molecule
A molecule that has a net dipole moment due to the presence of polar bonds.
Reference links
Supplementary resources to enhance your learning experience.
