CHEMICAL BONDING AND MOLECULAR STRUCTURE
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Chemical Bonding
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome everyone! Today, we're diving into chemical bonding, which is crucial for understanding how different elements combine to form molecules. Can anyone tell me what they think a chemical bond is?

Isn't it the force that holds atoms together?

Exactly! A chemical bond is an attractive force between atoms or ions. Now, there are mainly two types of chemical bonds: ionic and covalent. Who can explain the differences?

I think ionic bonds are formed when electrons are transferred from one atom to another, while covalent bonds involve sharing electrons.

Great explanation! We can remember this with the acronym 'TEA' — Transfer for Ionic and Exchange for Covalent. Let's move to the next topic!
The Octet Rule and Lewis Structures
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's talk about the octet rule. What does this rule state?

It states that atoms tend to form bonds until they are surrounded by eight valence electrons.

Exactly! This rule helps explain why certain elements bond. Now, how do we visually represent this?

By using Lewis structures, right? They show how electrons are arranged around atoms.

Exactly! Each dot represents a valence electron. Let's practice drawing the Lewis structure for water, H2O.

I see, we place two hydrogen atoms around oxygen and share the electrons!

Fantastic! Remember, it’s all about achieving that stable arrangement. Now, let's move on.
Covalent Bonds and VSEPR Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

We’ve covered ionic and covalent bonds. Now let’s explore the concept of covalent bonds in depth.

Covalent bonds are formed when two atoms share electrons, right?

Perfect! And to predict the shape of these molecules formed by covalent bonds, we use VSEPR theory. Does anyone know what VSEPR stands for?

Valence Shell Electron Pair Repulsion!

Correct! This theory helps us determine the molecular geometry based on how electron pairs repel one another. Can anyone give me an example?

Water has a bent shape due to the lone pairs on oxygen!

Exactly! That’s a great observation. The lone pairs take up space and push the hydrogen atoms closer together, resulting in that bent shape.
Hybridization and Molecular Orbital Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about hybridization, which explains the shape of molecules better. Can anyone tell me what hybridization involves?

I think it involves mixing atomic orbitals to form new hybrid orbitals?

Correct! This is crucial for molecules like methane, where sp3 hybridization occurs. What about molecular orbital theory?

It describes how atomic orbitals combine to form molecular orbitals, right?

Exactly! And remember, the order of filling these orbitals follows specific principles. Can anyone recall those principles?

The Aufbau principle, Pauli exclusion, and Hund’s rule?

Great job! You’re all doing fantastic. Let’s summarize what we've discussed in this session.
Hydrogen Bonding
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Lastly, let’s highlight hydrogen bonds. What is a hydrogen bond?

It’s a weak attraction between a hydrogen atom bonded to an electronegative atom and another electronegative atom.

Excellent! Hydrogen bonds are crucial in substances like water. How do they affect the properties of water?

They contribute to water's high boiling point and surface tension!

Perfectly stated! Now you can see how chemical bonds influence molecular properties across various compounds.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section outlines various theories of chemical bonding, including the significance of the octet rule, the formation and types of covalent and ionic bonds, the prediction of molecular geometry using VSEPR theory, and an introduction to hybridization and molecular orbital theory.
Detailed
Chemical Bonding and Molecular Structure
Chemical bonding is essential for the formation of molecules from atoms. In this section, several fundamental concepts and theories are explained:
Key Theories of Chemical Bonding
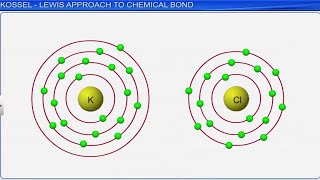
- Kössel-Lewis Approach: It provides a logical framework for understanding chemical bonds based on electron sharing. It emphasizes the importance of achieving a stable octet configuration in atoms similar to noble gases.
- Octet Rule: This rule states that atoms strive to achieve a full outer shell of eight electrons through gaining, losing, or sharing electrons to form stable compounds.
- Lewis Structures: Introduced by G.N. Lewis, these diagrams visually represent the bond formations and lone pairs in molecules, aiding in understanding molecular structure.
- Types of Bonds: The section distinguishes between ionic and covalent bonds, explaining their formation through electron transfer and sharing, respectively.
- VSEPR Theory: The Valence Shell Electron Pair Repulsion (VSEPR) theory predicts the geometry of molecules based on electron pair repulsion, helping in visualizing molecular shapes.
- Hybridization: This concept describes how different atomic orbitals mix to form new hybrid orbitals. It helps explain molecular shapes and bonding characteristics in complex molecules.
- Molecular Orbital Theory: Developed by F. Hund and R.S. Mulliken, this theory details the formation of molecular orbitals from atomic orbitals, highlighting how these orbitals influence molecular stability and electronic structure.
- Hydrogen Bonding: The section concludes with an explanation of hydrogen bonds, showcasing their role in determining the structure and properties of molecules like water and various organic compounds, differentiating between intermolecular and intramolecular hydrogen bonds.
Overall, the section provides a comprehensive insight into the fundamental aspects of chemical bonding and molecular structure, emphasizing their significance in chemistry.
Youtube Videos
![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Chemical Bonds
Chapter 1 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Matter is made up of one or different type of elements. Under normal conditions no other element exists as an independent atom in nature, except noble gases. However, a group of atoms is found to exist together as one species having characteristic properties. Such a group of atoms is called a molecule. Obviously, there must be some force which holds these constituent atoms together in the molecules. The attractive force which holds various constituents (atoms, ions, etc.) together in different chemical species is called a chemical bond.
Detailed Explanation
Chemical bonds hold molecules together. A molecule is a group of atoms bonded together, and the attractive forces between the atoms create stability in the structure. In nature, most elements do not exist independently as single atoms, with noble gases being a notable exception since they can exist alone due to their complete outer electron shell.
Examples & Analogies
Think of molecules like a team sports group where each player is an atom. Just like how teamwork (the bond) allows the players (atoms) to function together effectively, chemical bonds allow atoms to combine to create different substances. Without bonds, the players would not be able to form a team and play together.
Kössel-Lewis Approach to Bonding
Chapter 2 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In order to explain the formation of chemical bond in terms of electrons, a number of attempts were made, but it was only in 1916 when Kössel and Lewis succeeded independently in giving a satisfactory explanation. They were the first to provide some logical explanation of valence which was based on the inertness of noble gases...
Detailed Explanation
Kössel and Lewis established a theory to describe how atoms bond by focusing on the role of electrons, particularly the outer shell electrons known as valence electrons. They noticed that noble gases, which are very stable and do not readily form compounds, have a complete outer shell of electrons. Therefore, atoms strive to achieve the same stable configuration by gaining, losing, or sharing electrons to fill their outer shells, forming chemical bonds in the process.
Examples & Analogies
Imagine a jigsaw puzzle where the final picture represents stability. The pieces represent atoms that need to connect (bond) in a certain way to achieve the complete picture. Just like how you may need to exchange or rotate jigsaw pieces to complete the image, atoms must gain, lose, or share electrons to achieve a stable arrangement.
Octet Rule and Its Significance
Chapter 3 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Kössel and Lewis in 1916 developed an important theory of chemical combination between atoms known as the electronic theory of chemical bonding. According to this, atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an octet in their valence shells. This is known as the octet rule.
Detailed Explanation
The octet rule states that atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons, achieving a stable electronic configuration resembling that of noble gases. This rule helps to predict the types of chemical bonds that can form between different elements based on their electron arrangements.
Examples & Analogies
Consider a group of friends (the atoms) trying to form a circle (the stable octet). Each friend holds a certain number of hands (electrons) to connect with other friends. The goal is to form a complete circle with everyone holding hands. Some friends may need to give away hands or borrow hands to ensure that everyone is connected, which illustrates how atoms form bonds to achieve stability.
Covalent Bonds
Chapter 4 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Langmuir refined the Lewis postulations by abandoning the idea of the stationary cubical arrangement of the octet, and by introducing the term covalent bond. The formation of the Cl2 molecule can be understood in terms of the sharing of a pair of electrons between the two chlorine atoms.
Detailed Explanation
A covalent bond is formed when two atoms share one or more pairs of electrons. This sharing allows each atom to attain stability, as they achieve a complete octet arrangement. For example, in a chlorine molecule (Cl2), each chlorine atom contributes one electron to form a shared pair, resulting in a covalent bond that holds the two atoms together.
Examples & Analogies
Think of making a partnership in a project. If two people (the chlorine atoms) each contribute resources (electrons), they can work together efficiently, achieving a common goal (a stable bond). This partnership allows them to pool their assets (shared electrons) and successfully complete the project (create a stable Cl2 molecule).
Lewis Structures
Chapter 5 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule. While such a picture may not explain the bonding and behavior of a molecule completely, it does help in understanding the formation and properties of a molecule to a large extent.
Detailed Explanation
Lewis structures visually represent the arrangement of atoms in a molecule and indicate how electrons are shared and where lone pairs exist. These diagrams simplify the complex interactions of electrons and make it easier to predict chemical behaviors and reactivities of different substances.
Examples & Analogies
Imagine an architectural blueprint of a house. Just like the blueprint shows where each room (atom) is located and how the corridors (bonds) connect them, a Lewis structure provides a clear visual representation of how atoms are connected in a molecule and where the electrons are 'living' within that molecule.
Covalent Bond Strength and Multiple Bonds
Chapter 6 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
When two atoms share one electron pair, they are said to be joined by a single covalent bond. In many compounds, we have multiple bonds between atoms, which involves sharing more than one electron pair.
Detailed Explanation
Covalent bonds can be single (one pair of electrons shared), double (two pairs shared), or triple (three pairs shared). Multiple bonds are generally stronger and shorter than single bonds due to increased electron sharing between the bonded atoms. For instance, in carbon dioxide (CO2), each carbon atom forms a double bond with oxygen atoms.
Examples & Analogies
Think of a friendship. A single bond is like a casual friendship where you share occasional chats. A double bond represents a closer friendship where you share personal secrets. A triple bond is akin to an unbreakable bond where you share everything. The stronger and tighter the bond, the shorter and more intense the 'connection' becomes as you invest more with each 'pair' of shared moments.
Limitations of the Octet Rule
Chapter 7 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The octet rule, though useful, is not universal. It is particularly applicable to the second period elements of the periodic table. There are exceptions to this rule including molecules with incomplete octets, odd-electron molecules, and expanded octets.
Detailed Explanation
While the octet rule aids in predicting the structure of many molecules, it fails in specific cases. Some elements, like boron and aluminum, do not achieve eight electrons around them, while others with odd numbers of total electrons cannot satisfy the octet for all atoms. Additionally, elements below the second period can have more than eight electrons due to available d orbitals.
Examples & Analogies
Consider a rule at a club that states everyone must wear matching outfits (the octet). However, some members (like boron) may show up in casual wear (not achieving octet), and others (like phosphorus) might wear accessories (expanded octet). The rule applies to most but not to all, showcasing that sometimes flexibility in rules is necessary to accommodate different situations.
VSEPR Theory and Molecular Geometry
Chapter 8 of 8
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The VSEPR theory provides a way to predict the shapes of molecules based on electron repulsions. It considers both bonding and non-bonding electron pairs around a central atom, establishing that electron pairs will arrange themselves to minimize repulsion.
Detailed Explanation
According to VSEPR (Valence Shell Electron Pair Repulsion) theory, the shape of a molecule is determined by the repulsion between all electron pairs surrounding a central atom, including both bonded and lone pairs. By visualizing these repulsions, one can determine molecular shapes; for instance, tetrahedral shapes arise when four pairs maximize their distance from each other.
Examples & Analogies
Think of a crowded room where people (electron pairs) are trying to stay comfortable. They will naturally spread out to avoid bumping into one another (minimizing repulsion), forming a circle (geometry of a molecule). Each arrangement is determined by how many friends (electron pairs) are joining in and their interactions with each other.
Key Concepts
-
Chemical Bond: The attractive force between atoms.
-
Ionic and Covalent Bonds: Transfer vs. sharing of electrons.
-
Octet Rule: Stability through full outer electron shells.
-
Lewis Structures: Visual representation of molecule bonding.
-
VSEPR Theory: Geometry prediction through electron pair repulsion.
-
Hybridization: Formation of new orbital types for bonding.
-
Molecular Orbital Theory: Combining atomic orbitals for molecular formation.
-
Hydrogen Bonds: Important weak interactions influencing properties.
Examples & Applications
Water (H2O) forming with bent molecular geometry due to lone pairs on oxygen.
Sodium chloride (NaCl) showcasing an ionic bond through electron transfer.
Oxygen molecule (O2) exhibiting a double bond using molecular orbital theory.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Atoms seek to bond and thrive, with eight, they feel alive!
Stories
Once upon a time, lonely atoms joined forces, sharing electrons to find their stability and fill their shells, creating a happy molecule.
Memory Tools
Remember the order: 'Loves Are Better Always' for Lewis structures, Octet rule, Bonding vs. Covalent, Affected geometries.
Acronyms
HOBOS for remembering
Hydrogen bonding
Octet rule
Bond types
Orbital hybrids
Stable molecules.
Flash Cards
Glossary
- Chemical Bond
The attractive force that holds atoms together in a molecule.
- Ionic Bond
A type of bond formed through the transfer of electrons from one atom to another.
- Covalent Bond
A bond formed by the sharing of electron pairs between atoms.
- Octet Rule
A rule stating that atoms gain, lose, or share electrons in order to have eight electrons in their outer shell.
- Lewis Structure
A diagram that represents the valence electrons of atoms within a molecule.
- VSEPR Theory
A theory that predicts the geometry of molecules based on repulsions between electron pairs.
- Hybridization
The mixing of atomic orbitals to form new hybrid orbitals for bonding.
- Molecular Orbital Theory
A theory that describes molecular bonding as the combination of atomic orbitals to form molecular orbitals.
- Hydrogen Bond
A weak bond formed between a hydrogen atom and an electronegative atom.
Reference links
Supplementary resources to enhance your learning experience.
