Lattice Enthalpy
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Lattice Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are discussing lattice enthalpy, which is essential in understanding ionic compounds. It is the energy needed to separate one mole of an ionic solid into gaseous ions.

Why is it important to know about lattice enthalpy?

Great question! Understanding lattice enthalpy helps us measure the stability of ionic compounds. For example, NaCl has a lattice enthalpy of 788 kJ/mol.

Does that energy stay fixed for every ionic compound?

Not exactly! It varies based on the size and charge of the ions involved. Remember: size matters! Larger ions generally lead to lower lattice enthalpy.

What happens if the ions are charged more?

Excellent query! Greater charges lead to higher lattice enthalpy because of stronger electrostatic attraction.

In summary, lattice enthalpy tells us how strong the ionic bonds are in a solid!
Factors Affecting Lattice Enthalpy
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's explore factors affecting lattice enthalpy. We have ionic charge and radius.

So, higher charge equals higher energy required?

Correct! The more charged the ions, the stronger the attractive forces. Remember: Charge and distance—think of 'Courage!' for Charge and Radius!

How does distance make a difference?

Excellent point! As ions get bigger, they are further apart, which decreases the force between them. This is known as the 'Coulomb's law.' So larger ions result in lower lattice enthalpy.

Are there any exceptions?

Always stay curious! Some specific structures can lead to unusual results, but generally, these are the main factors. In summary: smaller, more highly charged ions create higher lattice enthalpy.
Understanding Crystal Geometry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To compute lattice enthalpy accurately, we must consider crystal geometry.

What do you mean by crystal geometry?

Great observation! Since ionic solids form 3D arrangements, the positions of ions play a huge role in how they interact. This complexity means we can't just add up attractive and repulsive forces.

So, we need a specific method to calculate it?

Exactly! Methods like Born-Haber cycles help in assessing lattice enthalpy, incorporating various thermodynamic data. Crystal structure indeed impacts calculations!

Can you give us an example?

Sure! To find NaCl's lattice enthalpy using experimental data can reveal involved energy processes, such as enthalpies of formation, leading us back to lattice enthalpy. In summary, geometry is crucial in this whole picture.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
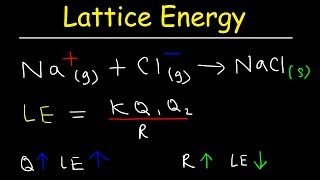
The lattice enthalpy quantifies the energy required to completely dissociate one mole of an ionic compound, as exemplified by NaCl needing 788 kJ/mol to separate into Na+ and Cl- ions. This concept integrates both the attractive and repulsive forces within the three-dimensional ionic crystal structure.
Detailed
Lattice Enthalpy
The lattice enthalpy of an ionic solid is defined as the energy required to separate one mole of a solid ionic compound into gaseous constituent ions. For instance, the lattice enthalpy of NaCl is 788 kJ/mol, indicating that this amount of energy is required to dissociate one mole of NaCl into gaseous Na+ and Cl- ions at an infinite distance.
This process involves two significant forces: the attractive interactions between ions of opposite charges and the repulsive interactions among ions of like charges. However, calculating lattice enthalpy directly from these forces is not feasible due to the complexities of a three-dimensional crystal lattice, which necessitates consideration of the crystal geometry as well.
Youtube Videos






![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Lattice Enthalpy
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The Lattice Enthalpy of an ionic solid is defined as the energy required to completely separate one mole of a solid ionic compound into gaseous constituent ions. For example, the lattice enthalpy of NaCl is 788 kJ mol–1. This means that 788 kJ of energy is required to separate one mole of solid NaCl into one mole of Na+ (g) and one mole of Cl– (g) to an infinite distance.
Detailed Explanation
Lattice enthalpy is a measure of the strength of an ionic bond in a compound. When ionic compounds, like NaCl, form, the ions attract each other due to their opposite charges. Breaking apart these ions requires energy, known as lattice enthalpy. For NaCl, this value is 788 kJ/mol, indicating it takes a significant amount of energy to separate the ions. This separation highlights how strongly they are held together in the solid state.
Examples & Analogies
Imagine trying to pull apart two magnets that are stuck together. The stronger the magnets, the more effort you must exert to separate them. In this analogy, the energy you use to separate the magnets represents the lattice enthalpy of an ionic compound. Just as the magnets have a powerful attraction due to magnetic forces, the ions in an ionic crystal are held tightly together by electrical forces.
Attractive and Repulsive Forces
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This process involves both the attractive forces between ions of opposite charges and the repulsive forces between ions of like charge.
Detailed Explanation
In the context of lattice enthalpy, when ions come together to form a solid ionic compound, they experience an attractive force due to their opposite charges (for example, Na+ and Cl-). However, when ions of the same charge come close to each other (like two Na+ ions), they repel each other. The lattice enthalpy reflects the balance of these attractive and repulsive forces: the stronger the attraction relative to the repulsion, the higher the lattice enthalpy.
Examples & Analogies
Think of a crowded party. Friends (positive ions) may want to come together and talk (attractive forces). However, two people who don't get along (like charges) will avoid each other, creating tension (repulsive force). The overall atmosphere (the lattice structure) depends on how many people get along and how many want to keep their distance. If most get along, the party (the lattice) is very stable, similar to a high lattice enthalpy.
Challenges in Measuring Lattice Enthalpy
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The solid crystal being three-dimensional; it is not possible to calculate lattice enthalpy directly from the interaction of forces of attraction and repulsion only. Factors associated with the crystal geometry have to be included.
Detailed Explanation
Lattice enthalpy cannot be simply calculated by looking at the forces between the ions, as the arrangement of these ions in a three-dimensional crystal structure also plays a critical role. The geometry of the crystal, specifically how the ions are arranged and the distances between them, significantly influences the overall energy required to separate them. Thus, advanced models and calculations are used to determine lattice enthalpy accurately, considering these geometric factors.
Examples & Analogies
Imagine trying to determine the strength of a 3D puzzle. If the pieces are snugly fit and well-shaped, the puzzle is stable (similar to high lattice enthalpy). However, if the pieces don't fit well, even if they’re close together, the puzzle can fall apart easily (low lattice enthalpy). Understanding how well the pieces fit and their arrangement is key to evaluating stability, much like the crystal structure in ionic solids.
Key Concepts
-
Lattice Enthalpy: Energy required to separate ionic compounds into individual ions.
-
Attractive Forces: Forces pulling oppositely charged ions together.
-
Repulsive Forces: Forces pushing like-charged ions apart.
-
Crystal Geometry: The arrangements of ions in a three-dimensional structure.
Examples & Applications
The lattice enthalpy of NaCl is 788 kJ/mol, illustrating the energy required for its dissociation.
For MgO, which has magnesium and oxygen ions, the lattice enthalpy is higher due to the +2 charge of magnesium.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Bonds so tight, need energy bright; to break apart, it’s a lattice art!
Stories
Imagine ionic knights locked in an embrace, needing a powerful force to break the solid chain and set the ions free.
Memory Tools
S-C (Size and Charge) for Lattice Enthalpy: Remember Size is bigger for low energy, Charge is stronger for high energy.
Acronyms
Remember the acronym 'LACE' - Lattice Energy, Attractive forces, Charges of ions, Effect of size.
Flash Cards
Glossary
- Lattice Enthalpy
Energy required to completely separate one mole of a solid ionic compound into gaseous ions.
- Ionic Compound
A compound composed of ions held together by ionic bonds.
- Electrostatic Attraction
The force that attracts oppositely charged ions towards each other.
- Coulomb's Law
A law stating that the force between two charged objects is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
- BornHaber Cycle
A thermodynamic cycle that relates the lattice energy of an ionic solid to other properties of the solid.
Reference links
Supplementary resources to enhance your learning experience.
