BONDING IN SOME HOMONUCLEAR DIATOMIC MOLECULES
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Homonuclear Diatomic Molecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore homonuclear diatomic molecules, which are molecules made up of two identical atoms, like hydrogen. Can anyone tell me what a homonuclear diatomic molecule is?

It’s a molecule that consists of two of the same type of atoms.

Exactly! In a molecule like H2, we have two hydrogen atoms. Can you tell me how they bond together?

I think they bond by sharing electrons.

That's right! This sharing occurs as a result of the overlap of their atomic orbitals. In hydrogen, this is specifically the 1s orbital. This overlap leads to a stable bonding molecular orbital, which lowers the potential energy. Remember, stable configurations correspond to lower energy!
Bonding in H2
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dive deeper into H2. Can anyone tell me what the electronic configuration of hydrogen is when it forms H2?

It should be (σ1s)², since there are two electrons in the bonding molecular orbital.

That’s correct! And what does this tell us about the bond order in H2?

The bond order is 1, which means there is one single covalent bond.

Exactly! And given that there's no unpaired electron in H2, it's also diamagnetic. Let's summarize this: H2 is held by a single bond with a bond dissociation energy of 438 kJ/mol and a bond length of 74 pm. Great job, everyone!
Bonding in Other Homonuclear Diatomic Molecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's look at the helium molecule, He2. Can anyone share the reason why He2 does not actually exist?

Because its bond order is 0, right? It has equal numbers of bonding and antibonding electrons!

Exactly! Now, how about Li2? Who can tell me its successful bond order?

Li2 has a bond order of 1 and is therefore stable. Its configuration is (σ1s)²(σ*1s)²(σ2s)².

Correct! And what about C2? How does its bonding differ from Li2?

C2 has a bond order of 2, which means it has a double bond, with its electrons in two pi orbitals.

Exactly! Therefore, C2 is more stable than Li2, showing how different bonding can impact molecular properties.
Oxygen and its Unique Properties
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s turn our attention to O2. What do we know about its electronic configuration?

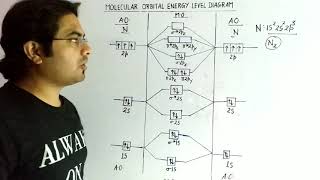
O2 has the configuration (σ1s)²(σ*1s)²(σ2s)²(σ*2s)²(σ2pz)²(π2px² = π2py²) and it has two unpaired electrons.

Great! Now, considering the unpaired electrons, what can we say about the magnetic properties of O2?

O2 is paramagnetic because of those unpaired electrons!

Exactly! The bond order is 2, indicating that O2 has a double bond and is essential for various biological processes.
Summary of Key Concepts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To wrap up today’s session, can someone summarize what we learned about bonding in homonuclear diatomic molecules?

We learned about the bonding in H2, He2, Li2, C2, and O2, focusing on their electronic configurations, bond orders, and stability.

And the significance of molecular orbitals was emphasized in determining magnetic properties.

Excellent! Remember the key concepts of bond order, stability, and paramagnetism as we move forward. Great job, everyone!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section details the bonding processes in homonuclear diatomic molecules, illustrating how molecular orbitals form and their significance in determining the properties of molecules. It emphasizes the role of bond order in assessing stability, magnetism, and molecular behavior in molecules such as H2, He2, and O2.
Detailed
Detailed Summary
This section delves into the bonding in homonuclear diatomic molecules, which consist of two identical atoms. The bonding is explained using the molecular orbital theory, focusing on the formation of molecular orbitals through the linear combination of atomic orbitals (LCAO).
- Hydrogen Molecule (H2): The hydrogen molecule is formed by the overlap of 1s orbitals from two hydrogen atoms, leading to a stable bond with a bond order of 1. Its electronic configuration is (σ1s)². The bond dissociation energy is 438 kJ/mol, with a bond length of 74 pm.
- Helium Molecule (He2): Helium's electronic configuration leads to (σ1s)²(σ*1s)², resulting in a bond order of 0, indicating instability and that He2 does not exist under normal conditions.
- Lithium Molecule (Li2): In Li2, there are six electrons with a configuration of (σ1s)²(σ*1s)²(σ2s)², granting a bond order of 1. Li2 is therefore stable and diamagnetic.
- Carbon Molecule (C2): C2 has a total of twelve electrons, resulting in a bond order of 2 due to its configuration (σ1s)²(σ*1s)²(σ2s)²(π2px²=π2py²). This indicates a double bond.
- Oxygen Molecule (O2): Oxygen has sixteen total electrons, leading to the electronic configuration (σ1s)²(σ1s)²(σ2s)²(σ2s)²(σ2pz)²(π2px²=π2py²)(π2p1x=π2py¹), resulting in a bond order of 2 and exhibiting paramagnetism due to two unpaired electrons.
Through the examples discussed, key principles of bond formation, stability, and the role of molecular orbitals in homonuclear diatomic molecules are emphasized.
Youtube Videos

![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Hydrogen Molecule (H2)
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In this section we shall discuss bonding in some homonuclear diatomic molecules.
- Hydrogen molecule (H2): It is formed by the combination of two hydrogen atoms. Each hydrogen atom has one electron in 1s orbital. Therefore, in all there are two electrons in hydrogen molecule which are present in σ1s molecular orbital. So electronic configuration of hydrogen molecule is H2: (σ1s)².
The bond order of H2 molecule can be calculated as given below:
Bond order = ½ (2 - 0) = 1
This means that the two hydrogen atoms are bonded together by a single covalent bond. The bond dissociation energy of hydrogen molecule has been found to be 438 kJ mol⁻¹ and bond length equal to 74 pm. Since no unpaired electron is present in hydrogen molecule, therefore, it is diamagnetic.
Detailed Explanation
The hydrogen molecule (H2) is formed when two hydrogen atoms combine. Each hydrogen atom contributes one electron from its 1s orbital. When they bond, these electrons fill a molecular orbital called σ1s, creating a stable configuration. The bond order is calculated as one bond (since there are two bonding electrons and no antibonding electrons), indicating the strength of the bond. This bond is characterized by a bond dissociation energy of 438 kJ/mol and a bond length of 74 pm. The absence of unpaired electrons in H2 means it's diamagnetic, meaning it won't be attracted to a magnetic field.
Examples & Analogies
Think of a hydrogen molecule like two friends holding hands. Each friend (a hydrogen atom) has one hand (one electron) to hold each other. When they join hands (bond), they create a strong bond (single bond). Just like how friends can stick together (stable bond), two hydrogen atoms stick together to form H2. The energy needed to separate them is like the energy needed for friends to break apart.
Helium Molecule (He2)
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Helium molecule (He2): The electronic configuration of helium atom is 1s². Each helium atom contains 2 electrons, therefore, in He2 molecule there would be 4 electrons. These electrons will be accommodated in σ1s and σ*1s molecular orbitals leading to electronic configuration:
He2: (σ1s)² (σ*1s)²
Bond order of He2 is ½ (2 - 2) = 0. He2 molecule is therefore unstable and does not exist.
Detailed Explanation
The helium molecule (He2) consists of two helium atoms, each with 2 electrons in the 1s orbital. When combined, these electrons fill both the bonding and antibonding molecular orbitals: σ1s and σ*1s. The bond order is calculated as 0 because the number of electrons in the bonding orbital equals the number in the antibonding orbital, indicating that there is no stable bond formed, hence He2 doesn't exist as a molecule.
Examples & Analogies
Imagine helium atoms like two balloons stuck together with one balloon tied to another balloon's string. When you fill both balloons with air (bonding electrons) and then let the air out (antibonding electrons), they deflate and can't hold on to each other anymore—that's why He2 can't exist as a stable molecule.
Lithium Molecule (Li2)
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Lithium molecule (Li2): The electronic configuration of lithium is 1s² 2s¹. There are six electrons in Li2. The electronic configuration of Li2 molecule, therefore, is:
Li2: (σ1s)² (σ*1s)² (σ2s)²
The above configuration is also written as KK(σ2s)² where KK represents the closed K shell structure (σ1s)² (σ*1s)².
From the electronic configuration of Li2 molecule it is clear that there are four electrons present in bonding molecular orbitals and two electrons present in antibonding molecular orbitals. Its bond order, therefore, is ½ (4 - 2) = 1. It means that Li2 molecule is stable and since it has no unpaired electrons it should be diamagnetic. Indeed diamagnetic Li2 molecules are known to exist in the vapour phase.
Detailed Explanation
The lithium molecule (Li2) consists of two lithium atoms, with one having a single electron in the outer shell. When lithium atoms bond, they create a configuration where there are four electrons in bonding orbitals (from the 1s and 2s levels) and two electrons in antibonding orbitals (from the 1s level). The bond order of 1 indicates a stable single bond, making Li2 diamagnetic as there are no unpaired electrons.
Examples & Analogies
Think of lithium atoms like two kids sharing a toy. The toy represents the shared bonding electrons. Each kid adds their own hand to hold onto the toy. With one toy firmly in their grasp, they're stable and happy, just like the Li2 molecule. But since the toy is shared, they don’t have any 'loose' hands (unpaired electrons)—thus, they're calm and 'quiet' like a diamagnetic material.
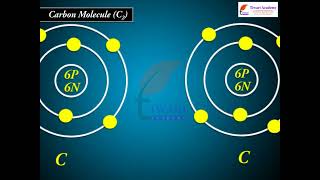
Carbon Molecule (C2)
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
- Carbon molecule (C2): The electronic configuration of carbon is 1s² 2s² 2p². There are twelve electrons in C2. The electronic configuration of C2 molecule, therefore, is
C2: (σ1s)² (σ*1s)² (σ2s)² (π2p²x = π2p²y)
or KK(σ2s)² (σ*2s)² (π2px² = π2py²).
The bond order of C2 is ½(8 - 4) = 2 and C2 should be diamagnetic. Diamagnetic C2 molecules have indeed been detected in vapour phase. It is important to note that double bond in C2 consists of both pi bonds because of the presence of four electrons in two pi molecular orbitals. In most of the other molecules a double bond is made up of a sigma bond and a pi bond.
Detailed Explanation
The carbon molecule (C2) has two carbon atoms, each contributing its electrons to form two pi bonds in addition to the sigma bonds. The bond order of 2 indicates a stable double bond configuration, consisting of both two pi bonds and a single sigma bond, which makes C2 relatively stable and diamagnetic as it has no unpaired electrons.
Examples & Analogies
Imagine carbon atoms like two friends sharing a strong friendship. Together, they create 4 layers of trust (bonds). Each layer represents a bond type; two pi bonds provide additional strength. Their bond is stable, much like a strong friendship that doesn’t let anyone else in. As they share all their resources (no unpaired electrons), they enjoy a tranquil, diamagnetic existence.
Oxygen Molecule (O2)
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
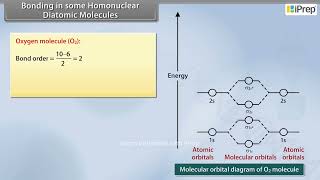
- Oxygen molecule (O2): The electronic configuration of oxygen atom is 1s² 2s² 2p⁴. Each oxygen atom has 8 electrons, hence, in O2 molecule there are 16 electrons. The electronic configuration of O2 molecule, therefore, is
O2: (σ1s)² (σ1s)² (σ2s)² (σ2s)² (σ2pz)² (π2px² ≡ π2py²) (π2px¹ ≡ π2py¹).
From the electronic configuration of O2 molecule it is clear that ten electrons are present in bonding molecular orbitals and six electrons are present in antibonding molecular orbitals. Its bond order, therefore, is:
Bond order = ½[Nb – Na] = [10 – 6] = 2.
So in oxygen molecule, atoms are held by a double bond. Moreover, it may be noted that it contains two unpaired electrons in π2px and π2py molecular orbitals, therefore, O2 molecule should be paramagnetic, a prediction that corresponds to experimental observation.
Detailed Explanation
The oxygen molecule (O2) is formed when two oxygen atoms combine, leading to a stable double bond configuration due to the filling of both bonding and antibonding molecular orbitals. The calculated bond order of 2 signifies that O2 is stabilized by the presence of both a sigma and two pi bonds while containing unpaired electrons, making it paramagnetic.
Examples & Analogies
Think of oxygen molecules as two dancers (oxygen atoms) twirling together in a ballet (bond). They hold onto each other securely (bonding electrons) while a bit of their energy escapes (unpaired electrons), causing them to dance with a little hover, making them energetic and paramagnetic.
Key Concepts
-
Homonuclear diatomic molecules consist of two identical atoms, like H2 or O2.
-
Bond order represents the number of chemical bonds between a pair of atoms.
-
Molecular orbitals are formed by the combination of atomic orbitals.
-
Paramagnetism arises from unpaired electrons in molecules.
Examples & Applications
The formation of H2 from two hydrogen atoms through the overlap of their 1s orbitals.
The non-existence of He2 due to a bond order of 0, indicating instability.
C2 bonds through two pi bonds, demonstrating a bond order of 2.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
To form a bond and stay aligned, share those electrons, and peace you'll find.
Stories
Once upon a time, H and H wanted to stick together, so they held hands and shared an electron to become H2!
Memory Tools
Remember: 'Hearts Bond Forever' to recall that bond order corresponds to stability.
Acronyms
HOMONO-DIATP
H2
He2
O2 — the bonding in homonuclear diatomic molecules.
Flash Cards
Glossary
- Homonuclear Diatomic Molecule
A molecule composed of two identical atoms.
- Bond Order
The number of bonds between a pair of atoms.
- Molecular Orbital
A region in a molecule where electrons are likely to be found.
- Paramagnetism
A property of a molecule that has unpaired electrons, making it attracted to a magnetic field.
Reference links
Supplementary resources to enhance your learning experience.
