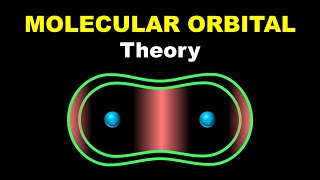
MOLECULAR ORBITAL THEORY
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Molecular Orbitals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss Molecular Orbital Theory, which explains how atomic orbitals combine and what molecular orbitals represent in a molecule's structure.

How do molecular orbitals differ from atomic orbitals?

Good question! While atomic orbitals are associated with single atoms, molecular orbitals are formed from the combination of atomic orbitals from two or more atoms. They can be bonding or antibonding, depending on how the atomic orbitals overlap.

What happens in bonding molecular orbitals?

In bonding molecular orbitals, electrons increase electron density between the nuclei, effectively stabilizing the molecule. Contrast this with antibonding orbitals, which have a node between the nuclei.

What do you mean by a node?

A node is a point in a molecule or orbital where the probability of finding an electron is zero. In antibonding orbitals, this node results in reduced attraction between nuclei.

Thanks for clarifying that!

Remember, molecular orbitals are crucial for understanding the stability and magnetic properties of molecules. Let's summarize our key points. Any questions?
Formation of Molecular Orbitals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, we will explore how molecular orbitals are formed using the Linear Combination of Atomic Orbitals, or LCAO method.

Can you explain how two hydrogen atoms form a molecular orbital?

Certainly! When two hydrogen atoms approach, their 1s atomic orbitals can combine through addition to form a bonding orbital and through subtraction to form an antibonding orbital.

What are the names of these orbitals?

The bonding orbital is called σ (sigma), and the antibonding orbital is σ* (sigma star). They are essential for representing the bonding interactions in molecules.

Why is the bond in hydrogen stable?

It's stable because the electrons in the σ bonding orbital hold the two nuclei together, effectively stabilizing the molecular structure.

Does this mean that for every two atomic orbitals, we create two molecular orbitals?

Exactly! And the total number of molecular orbitals corresponds to the number of atomic orbitals participating in the combination.

Before we finish, can anyone summarize what we learned today?

We learned about molecular orbitals formed through LCAO and the significance of bonding versus antibonding orbitals.
Bond Order and Stability
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To wrap up, let's discuss bond order and how it indicates the stability of molecules.

What exactly is bond order?

Bond order is defined as half the difference between the number of electrons in bonding and antibonding molecular orbitals. Higher bond order means greater stability.

Is it possible for a molecule to have a negative bond order?

Yes! A negative bond order implies that there are more electrons in antibonding orbitals than in bonding orbitals, indicating an unstable molecule.

What about practical examples?

Consider oxygen (O2), which has a bond order of 2 and is stable due to its double bond, while helium (He2), with a bond order of 0, does not exist.

So, bond order also helps determine if a molecule can exist?

Exactly! The concept of bond order ties the idea of electron configuration to molecular existence.

Thanks for the clear explanations!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section covers Molecular Orbital Theory, including the formation of molecular orbitals through the linear combination of atomic orbitals. It explains bonding in diatomic molecules, the significance of bonding and antibonding orbitals, and the implications for molecular stability, bond length, and magnetic properties.
Detailed
Molecular Orbital Theory
Molecular Orbital (MO) Theory, developed by F. Hund and R.S. Mulliken in 1932, provides a sophisticated way of understanding molecular bonding by considering the wave nature of electrons. In this theory:
- Electrons in Molecules: Electrons in a molecule occupy molecular orbitals, which are analogous to atomic orbitals but are influenced by the nuclei of both atoms in the molecule.
- Combination of Atomic Orbitals: Atomic orbitals of similar energy and symmetry combine to form molecular orbitals. For each pair of atomic orbitals that combine, two molecular orbitals are produced: one bonding and one antibonding.
- Characteristics of Molecular Orbitals:
- Bonding Orbitals: These have lower energy than the atomic orbitals from which they are formed and stabilize the molecule.
- Antibonding Orbitals: These are higher in energy and destabilize the molecule due to the presence of a node between the nuclei.
- Energy Level Diagrams: Molecular orbitals are filled based on their energy levels, following the Pauli Exclusion Principle and Hund’s rule.
- Stability and Bond Order: The stability of the molecule can be assessed through its bond order, defined as half the difference between the number of electrons in bonding and antibonding orbitals. A higher bond order indicates more stability.
- Examples of Homonuclear Diatomic Molecules: The application of MO Theory is illustrated with examples such as
- Hydrogen (H2): Forms a bonding molecular orbital (C3_{1s}) and has a bond order of 1.
- Oxygen (O2): Exhibits paramagnetic behavior due to unpaired electrons in its molecular orbitals.
- Nitrogen (N2): Demonstrates a bond order of 3 and is extremely stable.
Understanding Molecular Orbital Theory is essential for explaining the magnetic and stability properties of different molecular species and establishes a foundation for further exploration of chemical bonding.
Youtube Videos





![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Molecular Orbital Theory
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Molecular orbital (MO) theory was developed by F. Hund and R.S. Mulliken in 1932. The salient features of this theory are: (i) The electrons in a molecule are present in the various molecular orbitals as the electrons of atoms are present in the various atomic orbitals. (ii) The atomic orbitals of comparable energies and proper symmetry combine to form molecular orbitals.
Detailed Explanation
Molecular Orbital Theory provides insights into how atomic orbitals combine to form molecular orbitals. Unlike the earlier models that focused solely on bonds being a simple overlap of atomic orbitals, this theory recognizes that atomic orbitals can combine in ways that result in more complex interactions. The electrons in a molecule occupy these molecular orbitals, which can encompass contributions from multiple nuclei, leading to a more stable configuration.
Examples & Analogies
Think of molecular orbitals like a shared workspace where multiple people (electrons) can come together to collaborate on projects (bonding). Just as different workspaces allow for different kinds of collaboration, atomic orbitals combine in various ways to form molecular orbitals, creating stronger teams—or bonds.
Formation of Molecular Orbitals
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
According to wave mechanics, the atomic orbitals can be expressed by wave functions (ψ’s) which represent the amplitude of the electron waves. These are obtained from the solution of Schrödinger wave equation. To overcome the difficulty of directly obtaining molecular orbitals, an approximate method known as linear combination of atomic orbitals (LCAO) has been adopted.
Detailed Explanation
The Linear Combination of Atomic Orbitals (LCAO) is a critical method used in Molecular Orbital Theory. It allows for the formation of molecular orbitals by expressing atomic orbitals as wave functions. By adding or subtracting these wave functions, bonding and antibonding molecular orbitals are formed. This method highlights the importance of atomic orbital overlap in determining the properties of molecules.
Examples & Analogies
Imagine blending two different colors of paint. When you mix them together (adding the wave functions), you create a new color (molecular orbital). Sometimes, you might have a color that is more vibrant (bonding orbital) and sometimes a color that is dull (antibonding orbital), depending on how you mixed the paints.
Energy of Molecular Orbitals
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The bonding molecular orbital has lower energy and hence greater stability than the corresponding antibonding molecular orbital. The molecular orbitals like atomic orbitals are filled in accordance with the aufbau principle obeying the Pauli’s exclusion principle and the Hund’s rule.
Detailed Explanation
The stability and energy of molecular orbitals are fundamental concepts in molecular orbital theory. Bonding molecular orbitals, formed from the constructive overlap of atomic orbitals, have lower energy levels compared to antibonding orbitals, which form from destructive interference. This difference in energy levels explains why molecules tend to adopt configurations that maximize occupancy of bonding orbitals for stability.
Examples & Analogies
Think of bonding and antibonding orbitals like different levels in a swimming pool. The shallow end (bonding) is where everyone wants to be because it feels safe and comfortable. Conversely, the deep end (antibonding) is less stable and a bit more challenging, just like how filling antibonding orbitals leads to instability in molecules.
Types and Designation of Molecular Orbitals
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Molecular orbitals of diatomic molecules are designated as σ (sigma), π (pi), δ (delta), etc. In this nomenclature, the sigma (σ) molecular orbitals are symmetrical around the bond-axis while pi (π) molecular orbitals are not symmetrical.
Detailed Explanation
In molecular orbital theory, different types of molecular orbitals are described based on their geometric orientation. Sigma orbitals (σ) arise from end-to-end overlapping of atomic orbitals and are positioned symmetrically around the bond axis. Pi orbitals (π) arise from side-to-side overlaps and have different characteristics. This classification helps in understanding the bonding and shapes of various molecules.
Examples & Analogies
Consider the way bicycles are built. The main frame (σ bond) provides the crucial support and symmetry needed for stability in motion, while the extra wheels (π bonds) add character and support but are not as crucial for the core structure. This distinction helps in visualizing how different bonds can shape the stability and properties of a molecule.
Key Concepts
-
Molecular Orbital Theory: Explains how atomic orbitals combine to form molecular orbitals.
-
Bonding and Antibonding Orbitals: Determine the stability of molecules through electron occupancy.
-
Bond Order: Indicates the strength and stability of a bond.
-
Nodes: Points in molecular orbitals where electron probability is zero.
Examples & Applications
The hydrogen molecule (H2) forms by the overlap of 1s orbitals, resulting in a stable σ molecular orbital.
The oxygen molecule (O2) has unpaired electrons in its π* orbitals, resulting in paramagnetism.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In hydrogen's bond, we fondly see,
Stories
Imagine two friends named Sigma and Pi. Sigma loves to stick together through bonding, while Pi prefers to have some fun in the sky, floating between the friends but never truly sticking. Their relationship shows how atomic orbitals form the character of molecular orbitals.
Memory Tools
Remember 'BEA': Bonding electrons Add (to stability), Antibonding electrons Detract (from stability).
Acronyms
MO - 'My Orbital' refers to 'Molecular Orbital' representing collective electron behavior.
Flash Cards
Glossary
- Molecular Orbital
An orbital resulting from the combination of atomic orbitals that can contain electrons in a molecule.
- Bonding Orbital
A molecular orbital that stabilizes the molecule by concentrating electron density between the nuclei.
- Antibonding Orbital
A molecular orbital that destabilizes the molecule due to the presence of a node between the nuclei.
- Bond Order
A measure of bond strength calculated as half the difference between the number of electrons in bonding and antibonding orbitals.
- Node
A point in a molecular orbital where the probability of finding an electron is zero.
Reference links
Supplementary resources to enhance your learning experience.
