Formal Charge
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Formal Charge
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're diving into the concept of formal charge. Can anyone tell me what formal charge represents?

Is it how many electrons an atom has compared to when it's alone?

Exactly! The formal charge is the discrepancy between the number of valence electrons of an atom in isolation and the number assigned in the Lewis structure. It helps us understand electron distribution.

How do we calculate it?

Great question! We use the formula: Formal Charge = Valence electrons - Non-bonding electrons - 1/2(Bonding electrons). Let’s break that down further.

So, we consider all the electrons around the atom in the Lewis structure?

Precisely! By analyzing the number of bonding and non-bonding electrons, we can derive the formal charge, which helps us compare different structures to identify the most stable one.
Calculating Formal Charges
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's practice calculating formal charge. Consider the nitrate ion, NO3-. Who can walk me through the calculation for the nitrogen atom in this ion?

Nitrogen has 5 valence electrons in isolation.

In NO3-, nitrogen has one lone pair and three bonding pairs.

Correct! So how do we apply the formula?

Formal Charge = 5 - 2 - 3/2, which gives +1.

Excellent! This means nitrogen has a formal charge of +1. Remember, gaining stability is key in resonance structures!
Resonance and Formal Charge
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

How does the formal charge relate to resonance structures?

I guess it helps to find the most stable resonance form?

Right! Structures with lower or zero formal charge are generally more stable. Can anyone give me an example?

For ozone, O3. It has two resonance forms, and the formal charges help us identify the more stable one.

Correct! By calculating and comparing the formal charges, we can confirm which resonance structure is the most favorable.

So, is it always the case that the best structure has zero formal charge?

Not always! It frequently favors lower formal charge overall, leading us to structures that minimize charge separation.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Formal charge is defined as the difference between the total number of valence electrons of an atom in isolation and the number of electrons assigned to it in a Lewis structure. This concept plays a critical role in evaluating the most stable Lewis structure among potential resonance forms of a molecule.
Detailed
Formal Charge
Formal charge is a valuable concept in molecular chemistry, allowing chemists to assess the distribution of electrons among various atoms in molecular structures. It is calculated using the formula:
Formal Charge (F.C.) = Total valence electrons in the free atom - Total non-bonding electrons - (1/2) * Total bonding electrons
This formula illustrates how formal charge helps determine the stability of different resonance forms of a molecule by allowing chemists to identify which Lewis structure adheres closest to the principle of minimized formal charge for stability. The section addresses how formal charge calculation impacts individual atoms’ stability and bonding, assessing the appropriateness of Lewis structures and analyzing resonance.
Youtube Videos


![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Formal Charge
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The formal charge of an atom in a polyatomic molecule or ion may be defined as the difference between the number of valence electrons of that atom in an isolated or free state and the number of electrons assigned to that atom in the Lewis structure. It is expressed as:
Formal charge (F.C.) on an atom in a Lewis structure = total number of valence electrons in the free atom — total number of non-bonding (lone pair) electrons — (1/2) total number of bonding (shared) electrons.
Detailed Explanation
The formal charge gives us a way to assess how effectively an individual atom's valence electrons are being utilized in a molecule. By comparing the number of electrons an atom has in isolation with those it has in a molecular context, we can discern whether the atom is balanced, positively charged, or negatively charged within the structure.
The formula for calculating formal charge comprises three main parts:
1. Count the total number of valence electrons in the free atom.
2. Subtract the number of lone pair electrons that belong to that atom in the Lewis structure (these do not participate in bonding).
3. Subtract half of the number of shared bonding electrons (because each bond shares two electrons between two atoms, thus half reflects the contribution of that atom to the bond).
Examples & Analogies
Imagine you have a group project where different team members contribute different resources. Formal charge can be likened to figuring out how much effort each member put in versus what they initially committed to. If someone promised to contribute a lot but only offered a little, they would be seen as having a positive charge (as in more responsibility than is reflected), while someone who over-delivered on their commitment would show a negative charge (since they contributed more than expected).
Calculating Formal Charge
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
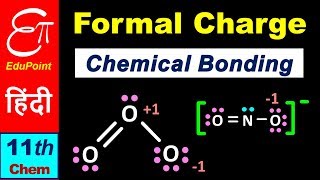
To illustrate how to calculate the formal charge, consider the ozone (O3) molecule. The formal charge on:
- The central O atom marked 1:
= 6 - 2 - (1/2 * 4) = +1
- The end O atom marked 2:
= 6 - 4 - (1/2 * 2) = 0
- The end O atom marked 3:
= 6 - 6 - (1/2 * 2) = -1
Thus, we represent O3 along with the formal charges as follows:
Detailed Explanation
Here’s how we apply the formula in context:
- For the central oxygen atom in ozone, we calculate how many valence electrons are counted in the Lewis structure versus how many it starts with. In this example, it has 6 valence electrons in an isolated state. In the Lewis structure, it has 2 electrons as lone pairs and 4 shared in bonds, leading to a +1 charge.
- The same process is applied to the other two oxygen atoms, where one ends up being neutral and the other carries a -1 charge. The distribution of formal charges helps identify which resonance structure would be most stable, as structures with the smallest total formal charge tend to be preferred.
Examples & Analogies
Consider the concept of credit and accountability in a group setting. If you’re responsible for getting some resources but you only manage to secure a few, you might have a positive balance of accountability (the formal charge). Conversely, if you take more responsibility than the group originally assigned to you, you might have a negative charge, indicating you’ve overachieved. This balance of responsibility is analogous to how atoms want to stabilize their charges.
Significance of Formal Charges
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Formal charges do not indicate real charge separation within the molecule. Instead, they help in keeping track of the valence electrons in the molecule and play a crucial role in selecting the lowest energy structure among several possible Lewis structures for a given species. Generally, the lowest energy structure is the one with the smallest formal charges on the atoms.
Detailed Explanation
The concept of formal charge plays a key role in molecular stability because it allows chemists to evaluate the most likely resonance structures. Though formal charges do not depict real charge distribution, they are vital for understanding the relative energies of various Lewis structures. The more balanced the charges (closer to zero), the more stable the configuration tends to be. This variability helps predict how molecules will behave chemically and physically, with less energy resources in a formal charge state resulting in lower energy configurations.
Examples & Analogies
Think of formal charges like a team balance sheet that tracks contributions and deficits. If each team member performs a task keeping their allocated duties in mind, the overall team performance is smoother and more efficient. However, if one member breaks this balance too much (similar to having high formal charges), the team may face instability, just like overly charged molecules may react or evolve differently than anticipated.
Key Concepts
-
Formal Charge: Calculated to determine stability.
-
Lewis Structures: Visual representation of bonding.
-
Resonance: Multi-structural representation for stability.
Examples & Applications
In the ozone molecule (
O3), the formal charge calculations help pinpoint the more stable resonance form.
When calculating the formal charge for nitrogen in NO3-, the value of +1 implies certain stability deviations compared to its base state.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
For formal charge, count the draw, valence first, that's the law!
Stories
Imagine a party where each atom needs to show how much they bring. Formal charge helps find who contributes what!
Memory Tools
F.C. = V.E. - N.B.E. - B.E.
Acronyms
FACES
Formal charge assesses charge electron stability.
Flash Cards
Glossary
- Formal Charge
The difference between the number of valence electrons in a free atom and the number assigned in the Lewis structure.
- Lewis Structure
A diagram showing the arrangement of valence electrons among the atoms in a molecule.
Reference links
Supplementary resources to enhance your learning experience.
