Energy Level Diagram for Molecular Orbitals
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Molecular Orbitals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will discuss molecular orbitals and how they form from atomic orbitals. When two atomic orbitals combine, they give rise to molecular orbitals. Can anyone tell me what happens during this process?

Do they combine to form two orbitals?

Exactly! When two atomic orbitals combine, they form one bonding molecular orbital and one antibonding molecular orbital. The bonding orbital is formed through constructive interference, giving stability to the molecule. Who can explain the significance of bonding and antibonding orbitals?

The bonding orbital has lower energy and contributes to stability, while the antibonding orbital has higher energy and makes the molecule less stable.

Great job! Remember to think of bonding orbitals as 'holding' atoms together. Let's use the acronym SPAM to summarize this: S for Stability, P for Pairing of Electrons, A for Atomic Overlap, and M for Molecular Orbitals.

What happens in a molecule with unpaired electrons?

Good question! If there are unpaired electrons in the antibonding orbitals, the molecule tends to be paramagnetic.

And if all orbitals are filled, it’s diamagnetic?

That's correct! Let's recap: bonding orbitals are stabilizing, antibonding orbitals destabilizing, and the presence of unpaired electrons can determine whether a molecule is paramagnetic or diamagnetic.
Energy Level Arrangement
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve into the energy level arrangement of molecular orbitals. When we analyze diatomic molecules, there is a specific order of energy.

What’s the typical energy order for these molecular orbitals?

For oxygen and fluorine, the order is: σ1s < σ*1s < σ2s < σ*2s < σ2pz < (π2px = π2py) < (π*2px = π*2py) < σ*2pz. Can someone explain why oxygen and nitrogen have a different order?

I think it's because of the additional electron repulsion in higher energy levels?

Exactly! This repulsion affects their energy arrangements. The bond order can be calculated from the difference between electrons in bonding and antibonding orbitals. If Nb is the number of electrons in bonding orbitals and Na is the number in antibonding, we can find bond order using: Bond order = (Nb - Na)/2.

So a higher bond order means a stronger bond?

You got it! To remember this, link 'bond order' with 'bond strength' as both increase together. Overall, the order and occupancy of these orbitals is critical for determining molecular stability and properties.
Applications of Molecular Orbital Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's summarize what we learned about molecular orbitals and discuss practical applications. Why do we care about knowing the molecular structure?

It helps in predicting chemical behavior, right?

Absolutely! For instance, understanding whether a molecule is paramagnetic can affect its interactions with external magnetic fields. Can you think of other examples where molecular orbitals are essential?

They help in understanding reaction mechanisms and stability!

Correct! The electronic configuration lets us predict bond lengths, energy, and even reactivity. Let's remember - stable structures often correlate with lower energy configurations. Always think of molecular orbitals as the gateway to understanding chemical properties.

So, the energy arrangements show stability and help with understanding molecular behavior!

Well done! Remember, the key relationship here is energy levels and their combined behavior in forming molecules.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The energy level diagram illustrates how atomic orbitals combine to form molecular orbitals, detailing the distinction between bonding and antibonding orbitals and their implications for molecular stability and properties.
Detailed
Energy Level Diagram for Molecular Orbitals
Molecular orbital (MO) theory provides a framework for understanding how atomic orbitals combine to form molecular orbitals in diatomic molecules. When atomic orbitals of the same or similar energy overlap, they form molecular orbitals which can be bonded (lower energy) or antibonded (higher energy).
Key Points:
- Formation of Molecular Orbitals: The linear combination of atomic orbitals (LCAO) leads to the formation of bonding (σ) and antibonding (σ*) orbitals.
- Energy Levels: The arrangement of these molecular orbitals is crucial for predicting molecular behavior, including stability and bond order.
- Order of Energy Levels: The energy order (e.g., σ1s < σ1s < σ2s < σ2s < σ2pz < (π2px = π2py) < (π2px = π2py) < σ*2pz) provides insight into the bond stability, where bonding orbitals lower the energy and antibonding orbitals increase it.
Understanding these concepts is vital for predicting molecular characteristics, including magnetism (paramagnetic versus diamagnetic behaviors) and bond energies. This section sets the groundwork for applying molecular orbital theory to homonuclear diatomic molecules, allowing simplification in predicting stability based on bond order and electronic configurations.
Youtube Videos


![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)


Audio Book
Dive deep into the subject with an immersive audiobook experience.
Formation of Molecular Orbitals
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
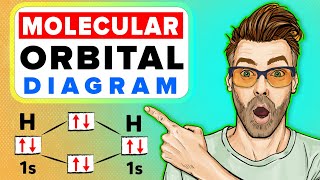
We have seen that 1s atomic orbitals on two atoms form two molecular orbitals designated as σ1s and σ*1s. In the same manner, the 2s and 2p atomic orbitals (eight atomic orbitals on two atoms) give rise to the following eight molecular orbitals:
Antibonding MOs σ2s σ2pz π2px π2py
Bonding MOs σ2s σ2pz π2px π2py
Detailed Explanation
This chunk discusses how molecular orbitals are formed from atomic orbitals. When atomic orbitals from two atoms (like hydrogen or any other element) combine, they create two distinct types of molecular orbitals: bonding and antibonding orbitals. The bonding orbitals lower the energy of the system and help stabilize the molecule, while the antibonding orbitals do the opposite and raise the energy level. For example, the 1s orbitals combine to form σ1s (bonding) and σ*1s (antibonding). Likewise, the 2s and 2p orbitals combine to form additional molecular orbitals.
Examples & Analogies
Think of forming molecular orbitals like creating a shared space between two rooms (atomic orbitals). If both rooms come together to create a larger room (bonding orbital), everyone in it feels more comfortable (lower energy). However, if a divider (antibonding orbital) is placed in the middle, it makes it cramped and uncomfortable (higher energy).
Energy Level Sequence of Molecular Orbitals
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
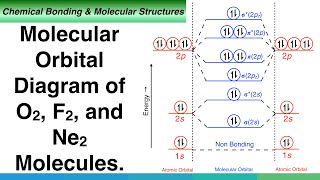
The energy levels of these molecular orbitals have been determined experimentally from spectroscopic data for homonuclear diatomic molecules of second row elements of the periodic table. The increasing order of energies of various molecular orbitals for O2 and F2 is given below:
σ1s < σ1s < σ2s < σ2s < σ2pz < (π2px=π2py) < (π2px=π2py) < σ*2pz
Detailed Explanation
This section provides a specific ordering of molecular orbitals according to their energy levels for oxygen (O2) and fluorine (F2) molecules. This order helps in understanding which molecular orbital is occupied first during the electron filling process. For instance, bonding molecular orbitals like σ2pz come before antibonding orbitals like σ*2pz, showing the preference for stability in filling lower-energy orbitals first.
Examples & Analogies
Imagine playing a game where players fill slots one by one but have to start from the lowest floor of a building first (lower energy). This ensures that the most stable areas of the building are filled before the more expensive penthouse suites (higher energy) are taken.
Molecular Orbital Configuration and Bond Order
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
However, this sequence of energy levels of molecular orbitals is not correct for the remaining molecules Li2, Be2, B2, C2, N2. For instance, it has been observed experimentally that for molecules such as B2, C2, N2, etc. the increasing order of energies of various molecular orbitals is:
σ1s < σ1s < σ2s < σ2s < (π2px=π2py) < σ2pz < (π2px=π2py) < σ*2pz.
Detailed Explanation
This portion highlights exceptions in the ordering of molecular orbitals, particularly for molecules such as B2, C2, and N2. Understanding these variations is vital as they impact the molecular stability and properties. The shifts in energy levels among these molecules dictate their unique chemical behaviors and interactions, illustrating the need for studying molecular orbital theory in depth.
Examples & Analogies
Consider a library with various sections (molecular orbitals). Most books (electrons) go into their specific categories (energy levels), but some books might go into less conventional sections based on their uniqueness. The library's layout (the energy distribution) changes as new sections become popular, similar to how these atoms interact.
Key Concepts
-
Bonding and antibonding orbitals impact molecular stability.
-
The LCAO method describes the formation of molecular orbitals.
-
Bond order relates directly to molecular stability and strength.
Examples & Applications
The molecular orbital configuration of O2 reveals that it is paramagnetic due to unpaired electrons.
For H2, the formation of a bonding molecular orbital results in a stable molecule with a bond order of 1.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Molecular orbitals bond and break, electrons dance, not a mistake!
Stories
Imagine two friends, A and B. They hold hands to make a stable bond, but if they try too hard and pull apart, they create tension and instability.
Memory Tools
BO=Nb-Na, bond order is what we see!
Acronyms
BAND for Bonding (Ba - Antibonding - Nb and Na Distribution)
Flash Cards
Glossary
- Bonding Molecular Orbital
An orbital where the electron density is between the nuclei, stabilizing the molecule.
- Antibonding Molecular Orbital
An orbital that has a region of zero electron density between the nuclei, destabilizing the molecule.
- Linear Combination of Atomic Orbitals (LCAO)
A method by which atomic orbitals combine to form molecular orbitals.
- Bond Order
The difference between the number of electrons in bonding and antibonding orbitals, divided by two, indicating the strength of the bond.
Reference links
Supplementary resources to enhance your learning experience.
