THE VALENCE SHELL ELECTRON PAIR REPULSION (VSEPR) THEORY
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Electron Pair Repulsion
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're diving into the VSEPR theory, which helps us predict the shapes of molecules based on the repulsions between electron pairs. Can anyone tell me what the term 'valence shell' refers to?

Is it the outermost shell of an atom where the electrons involved in bonding are located?

Exactly! Now, why do you think electron pairs repel each other?

Because they are both negatively charged?

Right! This repulsion leads to various molecular shapes. Remember the acronym 'VSEPR' - it stands for Valence Shell Electron Pair Repulsion. Let's explore the basic geometries associated with different numbers of electron pairs.
Molecular Shapes Based on Electron Pair Count
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

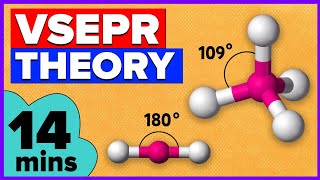
The first geometry is linear, which occurs when there are two bonding pairs and no lone pairs. Can you think of an example?

CO2 would be an example!

Correct! Next, let's discuss when we have three bonding pairs and no lone pairs. What shape does that create?

That would be trigonal planar.

Well done! Now, what happens if we introduce a lone pair? How does this affect the shape?

It makes the shape like a bent structure, right?

Exactly, especially in cases like water (H2O). Let's remember this pattern: Lone pair repulsion is stronger than bonding pair repulsion, which shifts the structure.
The Importance of Bond Angles
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about bond angles. If we have a tetrahedral shape, what is the expected bond angle?

It's about 109.5 degrees, right?

Correct! But when we have lone pairs, we just discussed how that can slightly distort this angle. What is our conclusion about lone pair effects?

Lone pairs push down the bond angles compared to ideal angles!

Precisely! Remember these adjustments in bond angles due to lone pairs.
Applications of VSEPR Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's consider how the VSEPR theory is applied in real life. Can someone tell me how molecular shapes influence their properties?

I think the shape affects how molecules interact scientifically, like in reactions or biological processes?

Spot on! However, the VSEPR model does have its limitations. Can anyone think of some?

It can't explain the actual shapes when resonant forms exist.

Great observation! And it cannot give us energy considerations. It’s a useful tool but has its boundaries.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
VSEPR theory emphasizes that the geometry of a molecule is predominantly determined by the repulsion between electron pairs in the valence shell. This theory uses basic principles of spatial orientation to predict distinct molecular shapes, highlighting the differences in repulsion among lone pairs and bonding pairs.
Detailed
The Valence Shell Electron Pair Repulsion (VSEPR) Theory posits that the molecular geometry of polyatomic substances is influenced by the repulsion between lone pairs and bonded pairs of electrons in the valence shell. The electron pairs tend to arrange themselves spatially to minimize repulsions, thereby maximizing their distances from one another. This theory simplifies bond geometry prediction by categorizing electron pair arrangements, resulting in versatile molecular shapes such as linear, trigonal planar, tetrahedral, and others based on the number of bonding and lone pairs around a central atom. Furthermore, it considers the greater spatial requirement of lone pairs, which leads to adjustments in expected bond angles and molecular shapes. Essentially, VSEPR theory serves as a critical tool in understanding the three-dimensional arrangements of atoms in molecules.
Youtube Videos





![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)
Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to VSEPR Theory
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As already explained, Lewis concept is unable to explain the shapes of molecules. This theory provides a simple procedure to predict the shapes of covalent molecules. Sidgwick and Powell in 1940, proposed a simple theory based on the repulsive interactions of the electron pairs in the valence shell of the atoms. It was further developed and redefined by Nyholm and Gillespie (1957).
Detailed Explanation
The VSEPR theory, which stands for Valence Shell Electron Pair Repulsion theory, was developed as a solution to limitations in Lewis structures, particularly regarding the prediction of molecular shapes. Sidgwick and Powell proposed this theory to account for the shape of molecules based on the repulsion between the electron pairs located in the outermost shell. This theory states that electron pairs (both bonding pairs shared by two atoms and lone pairs that belong to one atom) repel each other and will position themselves as far apart as possible to minimize this repulsion. Nyholm and Gillespie later refined this theory in 1957 to improve its explanatory power and to better classify molecular geometries.
Examples & Analogies
Think of electron pairs in a molecule like balloons in a room. If you filled a room with balloons, you'd want to spread them out as much as possible to prevent them from bumping into each other. Similarly, in a molecule, electron pairs want to be as far apart as possible, resulting in a specific shape that determines how the atoms in that molecule are arranged.
Key Postulates of VSEPR Theory
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The main postulates of VSEPR theory are as follows:
- The shape of a molecule depends upon the number of valence shell electron pairs (bonded or nonbonded) around the central atom.
- Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
- These pairs of electrons tend to occupy such positions in space that minimize repulsion and thus maximize distance between them.
- The valence shell is taken as a sphere with the electron pairs localizing on the spherical surface at maximum distance from one another.
- A multiple bond is treated as if it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair.
- Where two or more resonance structures can represent a molecule, the VSEPR model is applicable to any such structure.
Detailed Explanation
The VSEPR theory hinges on several key principles that dictate the geometrical arrangement of atoms in a molecule. First, the total number of electron pairs around a central atom influences its shape — more electron pairs lead to more interactions. Second, the repulsion between these negatively charged electron clouds influences how close they can get to each other. By occupying positions that minimize repulsion, the molecule assumes a specific shape. By visualizing the valence shell as a sphere, we can understand how these electron pairs maintain maximal distance from one another. Additionally, the theory treats multiple bonds like single electron pairs, simplifying the molecular geometry. Lastly, the VSEPR model remains applicable to any molecular structure that can be described through resonance.
Examples & Analogies
Imagine a group of friends holding hands to form a circle. If one friend has a hula hoop, they would naturally try to hold it away from the others while still keeping their hands linked. This situation illustrates how electron pairs in molecules behave, attempting to stay as far from each other as possible while still forming bonds with the central atom.
Electron Pair Repulsion Order
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The repulsive interaction of electron pairs decreases in the order:
- Lone pair (lp) – Lone pair (lp) > Lone pair (lp) – Bond pair (bp) > Bond pair (bp) – Bond pair (bp).
Detailed Explanation
This order of repulsion shows us that lone pairs exert the most repulsion. Since lone pairs are only associated with one atom, they occupy more space than bonding pairs, which are shared between two atoms. The increased repulsion from lone pairs affects the bond angles and shapes of molecules. The second form of repulsion occurs between lone pairs and bonding pairs, which still causes notable distortion in molecular geometry. Finally, bonding pairs only repel each other in influence, making their repulsion the least significant in shaping molecular geometry.
Examples & Analogies
Consider a dance party where couples (bond pairs) can dance with relative ease, but if a few friends (lone pairs) crowd the dance floor by themselves, they’re likely to get in each other’s way, causing confusion on the dance floor and affecting how the couples can move around. Similarly, in molecules, lone pairs can significantly influence how bonded atoms arrange themselves due to the strong repulsive forces they exert.
Predictions of VSEPR Theory
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The VSEPR Theory is able to predict the geometry of a large number of molecules, especially the compounds of p-block elements accurately. It is also quite successful in determining the geometry quite-accurately even when the energy difference between possible structures is very small.
Detailed Explanation
VSEPR theory has proven to be a valuable tool for predicting the molecular shape of various compounds, particularly those containing p-block elements, such as carbon and nitrogen. By using this theory, chemists can determine whether a molecule will be linear, trigonal planar, tetrahedral, etc., based purely on the arrangement of electron pairs around the central atom. Its predictions hold even when the theoretical calculations suggest only slight differences in structural forms, which may not have been evident otherwise.
Examples & Analogies
Think of VSEPR theory as a skilled architect who can predict the best design for a building based on the materials used and how much space is available. Just like the architect takes into account the arrangement and nature of each room to optimize space, chemists use VSEPR theory to understand how electron pairs arrange themselves around an atom to predict the stable molecular geometry.
Key Concepts
-
Electron pairs repel each other, influencing molecular shape.
-
The arrangement of electron pairs can be predicted using VSEPR theory.
-
Lone pairs exert greater repulsion than bonding pairs, altering bond angles.
Examples & Applications
Example 1: Water (H2O) has a bent shape due to the presence of two lone pairs on oxygen.
Example 2: Carbon dioxide (CO2) has a linear shape because of no lone pairs on carbon.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
VSEPR theory predicts where electrons go, to keep them far, arranging in a show!
Stories
Imagine electrons at a dance party, always pushing away from each other to keep enough personal space. This tells you how the molecular shape will look!
Memory Tools
Lone pairs are like extra guests at a party – they push things out and change the setup.
Acronyms
Use 'VSEP' to remember
Valence Shell Electron Pair.
Flash Cards
Glossary
- VSEPR Theory
A model used to predict the geometry of individual molecules based on the repulsion between electron pairs.
- Electron Pair
A pair of electrons occupying the same orbital.
- Lone Pair
A pair of valence electrons that are not shared with another atom.
- Bonding Pair
A pair of electrons shared between two atoms.
- Molecular Geometry
The three-dimensional arrangement of atoms within a molecule.
- Bond Angle
The angle formed between three atoms in a molecule.
Reference links
Supplementary resources to enhance your learning experience.
