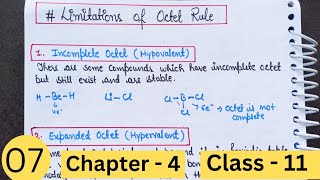
Limitations of the Octet Rule
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Incomplete Octets
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start our discussion on the limitations of the octet rule. Who can tell me what you understand by the term 'incomplete octet'?

Is it when atoms have fewer than eight electrons in their outer shell?

Exactly! Some elements, particularly those with less than four valence electrons, can form stable compounds without achieving an octet. Can you name a few examples?

I think lithium chloride, beryllium hydride, and boron trichloride would be examples.

Great job! Remember, Li, Be, and B are examples of elements that can have incomplete octets. It's crucial to understand these exceptions because they help explain the behavior of these compounds in chemical reactions.

So, these elements actually prefer fewer than eight electrons because it makes them more stable?

Exactly! It's a case of balancing electron configuration with stability, which defies the general application of the octet rule. Let's summarize: the incomplete octet applies particularly to elements with fewer than four valence electrons.
Odd-Electron Molecules
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss odd-electron molecules. What do you think happens in a molecule like nitric oxide, NO?

Does it mean that one of the atoms doesn't achieve an octet because there's an unpaired electron?

Right again! Molecules with an odd number of electrons cannot satisfy the octet rule for all atoms involved, leading to unpaired electrons. Can anyone think of another example?

I think nitrogen dioxide, NO₂, is another odd-electron molecule.

That's correct! These unpaired electrons can lead to interesting reactivity, so it's essential to consider odd-electron species in bonding.

So basically, the octet rule doesn't apply to these types of molecules?

Yes, and recognizing these exceptions helps in predicting molecular behavior and reactivity. Great discussion on odd-electron molecules!
Expanded Octets
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s talk about expanded octets. Why do you think elements like phosphorus and sulfur can have more than eight valence electrons?

Because they have d-orbitals available, right?

Precisely! Elements in and beyond the third period can utilize d-orbitals to accommodate more electrons. For example, what can you tell me about sulfur hexafluoride, SF₆?

It has twelve electrons around the sulfur atom when it forms bonds.

Correct! Understanding expanded octets is crucial as it directly influences the molecular geometry and bonding capabilities of these heavier elements. Let’s recap: expanded octets apply to elements in the third period and beyond, allowing them to have more than eight electrons.
Summary of Limitations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In summary, we’ve covered three main limitations of the octet rule: incomplete octets, odd-electron molecules, and expanded octets. Can anyone summarize why these are important to understand?

They help explain the behavior of compounds that don't conform to the basic rules in chemistry!

Exactly! Recognizing these limitations enhances our understanding of chemical bonding and molecular behavior. Keep these concepts in mind as they are crucial for predicting reactivity and stability in various compounds.
Real-world Applications
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss how these limitations apply in real-world chemistry. Can anyone think of a practical example where odd electron molecules have significant effects?

Yes, nitric oxide is important in the body as a signaling molecule!

Excellent! Its role as a signaling molecule in various physiological processes is a critical application of our understanding of odd-electron molecules. What about cases of expanded octets?

Maybe in coordination chemistry, where transition metals use expanded octets in complexing with ligands?

Exactly right! Transition metals often form complex ions where they utilize d-orbitals, leading to diverse chemistry. Keep these connections to real-world applications in mind as they reinforce your foundational understanding.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The octet rule is a foundational concept in chemistry that describes how atoms combine to achieve stability through having eight electrons in their valence shell. However, this rule has limitations, including cases of incomplete octets, situations involving molecules with an odd number of electrons, and the expanded octets observed in elements from the third period onwards. Understanding these exceptions is crucial for deeper comprehension of chemical bonding.
Detailed
Limitations of the Octet Rule
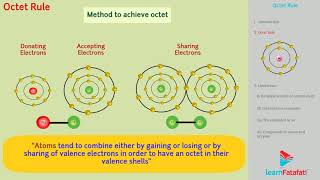
The octet rule provides a simplistic framework for understanding chemical bonding by stating that atoms tend to combine in ways that result in eight electrons in their outermost shell, similar to the noble gases. However, it is not a universal principle and exhibits several limitations:
- Incomplete Octets: Some elements can form stable compounds with fewer than eight electrons in their valence shell, commonly seen in elements with fewer than four valence electrons, such as lithium (Li), beryllium (Be), and boron (B). For instance, in compounds like Lithium Chloride (LiCl), Beryllium Hydride (BeH₂), and Boron Trichloride (BCl₃), the central atom does not achieve an octet.
- Odd-Electron Molecules: Molecules that contain an odd number of electrons, such as nitric oxide (NO) and nitrogen dioxide (NO₂), do not conform to the octet rule since there will be an unpaired electron in the structure, preventing all atoms in the molecule from achieving an octet.
- Expanded Octets: Elements in the third period (and beyond) have d-orbitals available for bonding and can have more than eight electrons in their valence shells. This is evidenced in compounds like phosphorus pentafluoride (PF₅), sulfur hexafluoride (SF₆), and sulfuric acid (H₂SO₄), where the central atom carries a total of more than eight electrons.
In cases like ozone (O₃), formal charges serve to indicate the stability of these structures, making it clear that the octet rule does not address all aspects of chemical bonding, particularly in complex molecules. It's essential to recognize these limitations to correctly predict molecular behavior and structure.
Youtube Videos







![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)
Audio Book
Dive deep into the subject with an immersive audiobook experience.
The Octet Rule's Applicability
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The octet rule, though useful, is not universal. It is quite useful for understanding the structures of most of the organic compounds and it applies mainly to the second period elements of the periodic table.
Detailed Explanation
The octet rule describes the tendency of atoms to prefer having eight electrons in their valence shell, similar to the electron configuration of noble gases. However, this rule is primarily applicable to the elements in the second period of the periodic table, like carbon, nitrogen, and oxygen. While it helps explain the bonding in many organic compounds, there are many cases, especially with other elements, where it does not hold true.
Examples & Analogies
Think of the octet rule like a house with eight rooms, where atoms want to fill all the rooms to feel comfortable. Most second-period elements are very particular about living in such houses, but some elements just don't care about filling all rooms and can be fine with fewer or more than eight 'occupants'.
Exceptions to the Octet Rule
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
There are three types of exceptions to the octet rule:
- The incomplete octet of the central atom
- Odd-electron molecules
- The expanded octet
Detailed Explanation
- Incomplete Octet: Some atoms such as lithium, beryllium, and boron can be stable with fewer than eight electrons. For example, beryllium typically forms compounds where it only has four electrons.
- Odd-electron Molecules: Molecules that have an odd number of total electrons cannot satisfy the octet rule for all atoms. For instance, in nitrogen dioxide (NO2), there are 11 total valence electrons, making it impossible for all atoms to have an octet.
- Expanded Octet: Starting from the third period of the periodic table, elements like phosphorus and sulfur can utilize d orbitals to accommodate more than eight electrons. For example, sulfur can have 12 electrons in its valence shell in compounds like SF6.
Examples & Analogies
Imagine you're organizing a party (electron configuration) and some of your friends (atoms) only want to invite a few guests (electrons). While most friends are happy with eight guests, some like, say, Boron, say, 'I’m only inviting three!' Meanwhile, friends like Sulfur are keen and say, 'I can handle twelve guests!' This showcases the flexibility and exceptions to what is often seen as a strict rule.
Understanding the Expanded Octet
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Elements in and beyond the third period of the periodic table have, apart from 3s and 3p orbitals, 3d orbitals also available for bonding. In a number of compounds of these elements, there are more than eight valence electrons around the central atom. This is termed as the expanded octet.
Detailed Explanation
The concept of the expanded octet comes into play with heavier elements that possess vacant d orbitals in addition to s and p orbitals. These orbitals can participate in bonding, allowing these elements to accommodate more electrons than those strictly dictated by the octet rule. Compounds like phosphorus pentafluoride (PF5) and sulfur hexafluoride (SF6) demonstrate this expanded capability.
Examples & Analogies
Think of it as adding extra chairs to a dining table. Regular guests (Atoms like carbon or nitrogen) fit comfortably with eight chairs, while heavier guests (like sulfur or phosphorus) can bring their own extra chairs (extra orbitals) to accommodate more friends (electrons) at the dinner table.
Formal Charge and Resonance
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Formal charges help in the selection of the lowest energy structure from a number of possible Lewis structures for a given species. Generally, the lowest energy structure is the one with the smallest formal charges on the atoms.
Detailed Explanation
Formal charge calculations help chemists identify the most stable resonance structures of molecules where more than one Lewis structure can be drawn. A formal charge is calculated based on the number of valence electrons, the number of bonds, and lone pairs. The most stable structure will have the lowest formal charge, leading to greater molecule stability.
Examples & Analogies
Imagine choosing the best version of a team logo (structure) to represent your group. You want one that looks good (stable) and doesn’t have too many design flaws (formal charges). Similarly, in chemistry, structures with low formal charges are preferred for their stability.
Other Drawbacks of the Octet Theory
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is clear that octet rule is based upon the chemical inertness of noble gases. However, some noble gases (for example xenon and krypton) also combine with oxygen and fluorine to form a number of compounds like XeF2, KrF2, XeOF2 etc. This theory does not account for the shape of molecules and does not explain the relative stability of the molecules being totally silent about the energy of a molecule.
Detailed Explanation
While the octet rule gives valuable insights into electron arrangements, it does not explain why some elements deviate from these idealizations, such as some noble gases forming compounds. Additionally, the theory lacks an explanation for molecular shapes and their bond angles, which are influenced by electron pair repulsion and spatial arrangements.
Examples & Analogies
Imagine that while planning a family gathering where everyone is expected to fit perfectly into eight chairs (octet rule). But then, some guests (like noble gases) surprise you by showing up and bringing their own extra chairs (form compounds), leading to a crowded room, highlighted by awkward positioning and discomfort (shapes/rules) that might not fit the original plan!
Key Concepts
-
Incomplete Octet: Refers to atoms with fewer than eight electrons, affecting stability and bonding.
-
Odd-Electron Molecules: Molecules that do not satisfy the octet rule for all atoms due to odd total electrons.
-
Expanded Octet: Atoms in the third period and beyond can accommodate more electrons than eight, using d-orbitals.
Examples & Applications
LiCl, BeH₂, and BCl₃ serve as examples of compounds with incomplete octets.
NO and NO₂ are classic examples of odd-electron molecules.
PF₅ and SF₆ illustrate compounds with expanded octets.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Octet rule's great, but sometimes it fails, with odd electrons, it pails.
Stories
Once there was a cowboy, who had many friends but could never find enough to make a full circle, like some atoms with fewer than eight electrons.
Memory Tools
I-O-E: Incomplete Octet, Odd-Electron molecules, Expanded Octet.
Acronyms
LOE for limitations of the octet
Less than eight
Odd-electrons
Expanded orbitals.
Flash Cards
Glossary
- Incomplete Octet
A situation in which an atom has fewer than eight electrons in its valence shell.
- OddElectron Molecules
Molecules that contain an odd number of electrons and therefore cannot satisfy the octet rule for all atoms.
- Expanded Octet
The phenomenon where atoms in the third period and beyond can utilize d-orbitals to accommodate more than eight electrons.
Reference links
Supplementary resources to enhance your learning experience.
