Summary
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Formation of Ions and Electrovalency
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss how ions form and what electrovalency means. Can anyone tell me why ions are important in chemical reactions?

Ions help in forming compounds, right?

Exactly! Ions achieve stability by attaining noble gas configurations. This process of stability is known as electrovalency. Can anyone explain how electrostatic attraction plays a role here?

Is it because oppositely charged ions attract each other?

Yes! That attraction is what stabilizes the ionic compounds. This concept is essential as it sets the foundation for understanding ionic bonds.
Covalent Bonding and Lewis Structures
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's shift gears to covalent bonding. What do you think defines a covalent bond?

Isn't it the sharing of electron pairs between atoms?

Perfect! This sharing allows atoms to achieve the same noble gas configuration. Lewis developed a method to represent this using dot structures. Can anyone explain what those structures show?

They show how many valence electrons are available for bonding?

Exactly! They illustrate both bonded and lone pairs of electrons. Understanding this is crucial for predicting molecular behavior.
Resonance and Molecular Geometry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we dive deeper, let's talk about resonance. Can someone explain why some molecules require multiple Lewis structures?

It’s when a single structure doesn't accurately represent the molecule?

Correct! These multiple structures are called canonical forms and together they create a resonance hybrid. This concept is important in understanding molecular shapes as well.

What helps determine those shapes?

Great question! The VSEPR model states that electrons repel each other, arranging themselves to minimize this repulsion. Can anyone recall the order of repulsion strength?

Yeah! Lone pairs repel more strongly than bonding pairs.

Exactly! lp-lp > lp-bp > bp-bp is the order to remember. Very well done!
Hybridization and Molecular Orbitals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To wrap this up, let’s discuss hybridization. What does hybridization tell us about molecular shapes?

It combines atomic orbitals to form new shapes for bonding?

Exactly! For example, sp3 hybridization explains the tetrahedral shape of CH4. Now, who can summarize what molecular orbital theory states?

It’s about combining atomic orbitals to create molecular orbitals that affect the molecule as a whole?

Correct! Remember, bonding orbitals have lower energy than atomic orbitals. Fantastic work today, everyone!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section highlights the mechanisms of ion formation relating to noble gas configurations, the nature of ionic and covalent bonds, and introduces critical theories such as VSEPR, valence bond, and molecular orbital theory. It emphasizes the impact of hybridization, resonance, and hydrogen bonding in explaining the properties and behavior of various compounds.
Detailed
Detailed Summary
The section begins with Kössel’s insight into ion formation, explaining that ions () attain stability by achieving noble gas configurations. This establishes the idea of electrovalency, where the electrostatic attraction between oppositely charged ions leads to stability.
The concept of covalent bonding is introduced, outlining Lewis's description of bonding as the sharing of electron pairs between atoms, again aimed at achieving noble gas configurations. This includes explanations on Lewis dot symbols and Lewis dot structures, which are important in illustrating valence electrons and bonding in various molecules.
Ionic compounds are depicted as ordered arrangements of ions in what is called a crystal lattice, which is stabilized by the enthalpy of lattice formation, maintaining charge balance between positively and negatively charged ions.
Covalent bonds differ from ionic bonds in that a single bond results from one shared electron pair, while multiple bonds result from sharing two or three pairs. Additionally, lone pairs—electron pairs not involved in bonding—are discussed alongside Lewis dot structures that visually represent their arrangement.
The section touches upon resonance, an essential concept for molecules and polyatomic ions that cannot be adequately described by a single Lewis structure but require multiple representations, known as canonical forms, to show their stable structure as a resonance hybrid.
The VSEPR model is discussed, emphasizing that the geometrical shapes of molecules are determined by electron pair repulsions, categorized in an order of decreasing strength. The valence bond (VB) theory complements this by discussing the energy dynamics during bond formation through orbital overlap, specifically highlighting the formation of diatomic hydrogen (H2).
Furthermore, Pauling’s concept of hybridization is brought forward to explain molecular shapes, involving the sp, sp2, and sp3 hybridizations critical for various molecules like BeCl2, CH4, and C2H4.
Lastly, the molecular orbital (MO) theory is explained, demonstrating how atomic orbitals combine to form molecular orbitals. This theory discusses the energy levels of bonding and antibonding orbitals along with the criteria for molecular stability based on electron configurations. Additionally, hydrogen bonding—both intermolecular and intramolecular—is summarized, illustrating its significance in influencing the properties and structures of many compounds.
Youtube Videos
![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)





Audio Book
Dive deep into the subject with an immersive audiobook experience.
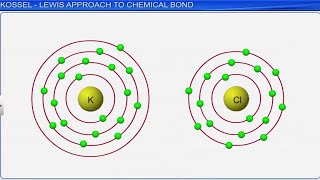
Kössel's Insights on Ion Formation
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Kössel's first insight into the mechanism of formation of electropositive and electronegative ions related the process to the attainment of noble gas configurations by the respective ions. Electrostatic attraction between ions is the cause for their stability. This gives the concept of electrovalency.
Detailed Explanation
Kössel identified that ions form to achieve a stable electron configuration similar to noble gases, which are chemically inert and stable. Electropositive atoms lose electrons to become positively charged, while electronegative atoms gain electrons to become negatively charged. The resulting attraction between positive and negative ions stabilizes the compound, making electrovalency an essential concept in understanding ionic bonding.
Examples & Analogies
Think of electrovalent bonding like a marriage. One partner gives up a little (an electron) to balance the needs of the other partner who receives it, creating a bond that is beneficial and stable for both. Just as relationships flourish on mutual support, ionic compounds gain stability through the strong attraction of oppositely charged ions.
Lewis's Description of Covalent Bonding
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The first description of covalent bonding was provided by Lewis in terms of the sharing of electron pairs between atoms and he related the process to the attainment of noble gas configurations by reacting atoms as a result of sharing of electrons. The Lewis dot symbols show the number of valence electrons of the atoms of a given element and Lewis dot structures show pictorial representations of bonding in molecules.
Detailed Explanation
Lewis introduced the concept that atoms share pairs of electrons to form covalent bonds, leading to a stable configuration akin to that of noble gases. He created symbols (Lewis dots) to visually represent the electron arrangement for each element. Covalent bonds can be visualized using Lewis dot structures, where dots represent valence electrons and lines represent bonds between atoms.
Examples & Analogies
Imagine sharing food in a potluck—each friend brings a dish (electron), and sharing creates a fuller meal (stable bond) that everyone enjoys. Just like in a potluck, where everyone contributes to create a satisfying meal, in a covalent bond, atoms share electrons to achieve stability.
Ionic Compounds and Crystal Lattices
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An ionic compound is pictured as a three-dimensional aggregation of positive and negative ions in an ordered arrangement called the crystal lattice. In a crystalline solid, there is a charge balance between positive and negative ions. The crystal lattice is stabilized by the enthalpy of lattice formation.
Detailed Explanation
Ionic compounds form structured arrangements called crystal lattices, where cations and anions are aligned in a way that maximizes the electrostatic attraction between them. Each positive ion is surrounded by negative ions and vice versa, ensuring stability. The stability of these structures is influenced by the energy released when the lattice forms, known as lattice enthalpy.
Examples & Analogies
Think of a crystal lattice like a well-ordered neighborhood where each house (ion) is perfectly placed to maximize safety and support (stability). Just as neighbors interact in a community to maintain structure and support, the positive and negative ions work together to form a strong, stable ionic compound.
Covalent Bonds and Multiple Bonds
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
While a single covalent bond is formed by sharing of an electron pair between two atoms, multiple bonds result from the sharing of two or three electron pairs. Some bonded atoms have additional pairs of electrons not involved in bonding. These are called lone pairs of electrons. A Lewis dot structure shows the arrangement of bonded pairs and lone pairs around each atom in a molecule.
Detailed Explanation
Covalent bonds can be single, double, or triple depending on how many pairs of electrons are shared between atoms. For example, a double bond involves two pairs of shared electrons. In addition to bonding pairs, some electrons remain unshared, called lone pairs. Lewis structures help visualize both bonded and unbonded electrons, providing a clearer picture of molecular structure.
Examples & Analogies
Consider a group of friends sharing tasks for a project. If one pair of friends (electron pairs) decides to do two tasks together, that’s like a double bond! Meanwhile, one friend might handle an undecided task alone (lone pair). Visually representing their roles in a project plan (Lewis dot structure) helps everyone understand their contributions.
Resonance Structures
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A number of molecules and polyatomic ions cannot be described accurately by a single Lewis structure, and a number of descriptions (representations) based on the same skeletal structure are written and these taken together represent the molecule or ion. This is a very important and extremely useful concept called resonance. The contributing structures or canonical forms taken together constitute the resonance hybrid which represents the molecule or ion.
Detailed Explanation
Resonance structures are multiple Lewis structures that represent a single molecule or ion which cannot be adequately depicted by just one structure. These representations exist because certain compounds exhibit characteristics of several possible structures. The actual structure is a hybrid of all these resonance forms, leading to increased stability.
Examples & Analogies
Think of resonance like a musician playing a song with variations. Each variation (resonance structure) represents a different interpretation of the same piece of music (molecule). No single variation captures the entire essence, but the combination of all variations creates a richer, more nuanced experience (the resonance hybrid).
Hydrogen Bonds and Their Types
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Hydrogen bond is formed when a hydrogen atom finds itself between two highly electronegative atoms such as F, O, and N. It may be intermolecular (existing between two or more molecules of the same or different substances) or intramolecular (present within the same molecule). Hydrogen bonds have a powerful effect on the structure and properties of many compounds.
Detailed Explanation
Hydrogen bonds are weak interactions that occur when hydrogen atoms are attracted to electronegative atoms (like fluorine, oxygen, or nitrogen) in other molecules. They can either form between different molecules (intermolecular) or within a single molecule (intramolecular). These bonds play a crucial role in determining the physical properties of substances like water, giving it special characteristics such as high boiling point and surface tension.
Examples & Analogies
Imagine friends working together on projects. If one friend depends on another to get help on their tasks (like a hydrogen bond), this connection might be weaker than a close partnership but still influences how they work together. This dependence leads to unique outcomes in their collaboration, much like how hydrogen bonding influences the unique properties of water.
Key Concepts
-
Electrovalency: The stability of ions based on achieving noble gas configurations.
-
Covalent Bonding: The sharing of electron pairs to form stable molecules.
-
Lewis Structures: Diagrams that show electron arrangements in molecules.
-
VSEPR Model: A theory used to predict molecular shapes based on electron repulsion.
-
Hybridization: The mixing of atomic orbitals to explain molecular geometry.
-
Molecular Orbital Theory: A method to describe molecular bonding using energy levels of orbitals.
Examples & Applications
Sodium chloride (NaCl) is an example of an ionic compound formed by the electrostatic attraction between Na+ and Cl- ions.
Methane (CH4) illustrates covalent bonding where carbon shares its electrons with four hydrogen atoms, adopting an sp3 hybridization.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
For ionic bonds, there’s a pull, opposite charges, feeling full.
Stories
Imagine a couple, one rich (positive ion) and one poor (negative ion), coming together for a stable relationship, forming a sweet ionic bond.
Memory Tools
For VSEPR, remember: 'Lone pairs repel most, then bonding pairs, they boast.'
Acronyms
R.E.A.C.T. — Resonance, Electron sharing, Attraction, Covalent bonds, and Theories of bonding.
Flash Cards
Glossary
- Electrovalency
The concept of ion stability based on electrostatic attraction between charged ions.
- Covalent Bonding
A type of chemical bond where electron pairs are shared between atoms.
- Lewis Dot Structure
A schematic representation used to show the valence electrons of an atom and the arrangement of electrons in a molecule.
- Resonance
A concept used for compounds that cannot be adequately described by a single Lewis structure but require multiple structures.
- Hybridization
The concept of mixing different types of atomic orbitals to form new hybrid orbitals for bonding.
- VSEPR Model
A model that predicts the geometrical structure of molecules based on electron pair repulsions.
- Molecular Orbital Theory
A theory that describes the electronic structure of molecules in terms of molecular orbitals formed from atomic orbitals.
Reference links
Supplementary resources to enhance your learning experience.
