Formation of Molecular Orbitals Linear Combination of Atomic Orbitals (LCAO)
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Molecular Orbitals and LCAO
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we're diving into how molecular orbitals are formed! Let’s start with the concept of Linear Combination of Atomic Orbitals, or LCAO. Can anyone remind me what atomic orbitals are?

I think atomic orbitals are regions around the nucleus where electrons are likely to be found.

Exactly! Now, when combining atomic orbitals, we can create molecular orbitals. What do you think happens when two atomic orbitals combine?

They can create new orbitals, right? Like bonding or antibonding orbitals.

Great insight! Remember, bonding molecular orbitals are formed when atomic orbitals combine in phase, reinforcing each other. On the flip side, antibonding orbitals are created when they combine out of phase, which actually destabilizes the molecule. To help remember this, think of the phrases 'Together' for bonding and 'Apart' for antibonding.

So, bonding orbitals help hold the molecule together while antibonding ones push it apart?

Exactly! Let’s recap that bonding MOs promote stability while antibonding MOs hinder it.
Understanding Energy and Symmetry in Orbital Overlap
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand bonding and antibonding, let’s explore the conditions necessary for effective orbital overlap. Can anyone suggest why energy levels matter?

I think if the energies are too different, they won’t combine well?

Exactly! The atomic orbitals must have similar energy for optimal overlap. Also, they need the same symmetry. What do you think that means?

Does it mean they should align in a way that allows effective bonding, like both being 'p' orbitals?

Yes, perfect! The atomic orbitals should also overlap maximally. We want our overlapping regions crowded with electrons to minimize repulsion.

So the more they overlap, the stronger the bond?

Exactly! Let’s summarize this: same energy, same symmetry, and maximum overlap are crucial!
Applications and Implications of Molecular Orbitals
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Alright! Now that we have the basics down, let's discuss how this impacts molecular stability. What can we infer about a molecule with more electrons in bonding than in antibonding orbitals?

It would be stable, right? Since bonding electrons promote stability!

Correct! It’s like having a solid foundation for a house. Now, does anyone know how we can determine the bond order from molecular orbitals?

Is it the difference between bonding and antibonding electrons divided by two?

Exactly! Bond order tells us the strength of a bond. Higher bond orders mean shorter, stronger bonds!

So, if we find out the bond order is negative, does that mean the molecule is unstable?

Yes! A negative or zero bond order indicates instability. Great predictions! Let’s summarize that: stable molecules have a greater number of bonding electrons than antibonding.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The formation of molecular orbitals involves the combination of atomic orbitals from two atoms using linear combination methods, yielding bonding and antibonding orbitals. Key principles include requirements for orbital symmetry and energy levels, which dictate how molecular orbitals provide stability to molecules.
Detailed
Formation of Molecular Orbitals
Molecular orbitals (MOs) are formed through the combination of atomic orbitals from individual atoms. This section discusses the formation of molecular orbitals using the Linear Combination of Atomic Orbitals (LCAO) method. The key concepts include:
- Wave Functions: Atomic orbitals are described by wave functions that represent the probability amplitude of electron positions in an atom, derived from solving the Schrödinger equation.
- Combination of Atomic Orbitals: Two essential types of combinations occur:
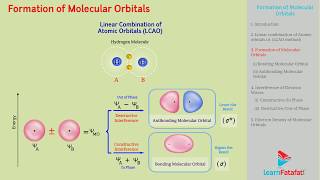
- Bonding Molecular Orbitals (σ): Formed when atomic orbitals combine in phase (constructive interference), resulting in increased electron density between nuclei, leading to a more stable arrangement.
- Antibonding Molecular Orbitals (σ*): Formed when atomic orbitals combine out of phase (destructive interference), resulting in a nodal plane and increased repulsion between nuclei, which destabilizes the molecule.
- Conditions for Combination: For effective combination, the atomic orbitals must:
- Have comparable energy levels.
- Share symmetry about the molecular axis.
- Overlap to the maximum extent.
- Types of Molecular Orbitals: MOs can be classified based on their symmetry around the bond axis:
- σ (sigma) MOs: Symmetrical around the bond axis.
- π (pi) MOs: Formed through side-by-side overlap, with electron density situated above and below the internuclear axis.
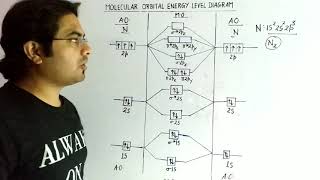
- Energy Level Diagrams: The energy levels of molecular orbitals are influenced by the types of atomic orbitals combined. The characteristics of MOs can be deduced from these energy arrangements, allowing for prediction of molecular stability.
Understanding molecular orbitals is critical for predicting the behavior of diatomic and polyatomic molecules, influencing concepts such as bond order, stability, and magnetic properties.
Youtube Videos



![Chemical Bonding and Molecular Structure [Complete] in Just 30 Minutes](https://img.youtube.com/vi/H1-COuLbvzI/mqdefault.jpg)



Audio Book
Dive deep into the subject with an immersive audiobook experience.
Linear Combination of Atomic Orbitals (LCAO)
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
According to wave mechanics, the atomic orbitals can be expressed by wave functions (ψ ’s) which represent the amplitude of the electron waves. These are obtained from the solution of Schrödinger wave equation. However, since it cannot be solved for any system containing more than one electron, molecular orbitals which are one electron wave functions for molecules are difficult to obtain directly from the solution of Schrödinger wave equation. To overcome this problem, an approximate method known as linear combination of atomic orbitals (LCAO) has been adopted.
Detailed Explanation
The LCAO method is a way to construct molecular orbitals by combining the atomic orbitals of constituent atoms. First, we express atomic orbitals as wave functions, which are mathematical representations of the electron's probable position and behavior. The LCAO approach allows us to combine these wave functions to create molecular orbitals, which describe electrons in a molecule rather than in separate atoms. The idea is that when two atomic orbitals combine, they can form new orbitals that can stabilize the molecule.
Examples & Analogies
Think of atomic orbitals as musical instruments. Each instrument (atomic orbital) has its own sound (energy level). When two musicians (atoms) decide to play together, they can create a new song (molecular orbital) that blends their sounds. If they play well together, their music (electrons in the molecule) becomes harmonious and creates a pleasant melody (bonding), but if they play against each other, the song becomes discordant (antibonding).
Formation of Bonding and Antibonding Orbitals
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let us apply this method to the homonuclear diatomic hydrogen molecule. Consider the hydrogen molecule consisting of two atoms A and B. Each hydrogen atom in the ground state has one electron in 1s orbital. The atomic orbitals of these atoms may be represented by the wave functions ψA and ψB. Mathematically, the formation of molecular orbitals may be described by the linear combination of atomic orbitals that can take place by addition and by subtraction of wave functions of individual atomic orbitals as shown below : ψMO = ψA + ψB Therefore, the two molecular orbitals σ and σ are formed as : σ = ψA + ψB ; σ = ψA – ψB.
Detailed Explanation
When the wave functions of two hydrogen atoms combine, two types of molecular orbitals are created: bonding and antibonding orbitals. The bonding orbital (σ) is formed by the addition of the two wave functions, leading to increased electron density between the two nuclei. This electron density helps hold the nuclei together, resulting in a stable, lower energy configuration. Conversely, the antibonding orbital (σ*) is formed by the subtraction of the wave functions, leading to a nodal plane where there is no electron density between the nuclei. This situation destabilizes the molecule and raises its energy.
Examples & Analogies
Imagine two friends pushing a swing at the same time (bonding orbital). When they push together, the swing goes higher (the energy is lower and more stable). Now, if one friend pushes forward and the other pulls back (antibonding orbital), they cancel each other's efforts, and the swing doesn’t go anywhere (unstable). The collaboration of the two friends, just like atomic orbitals, creates a strong or weak bond depending on how well they work together.
Conditions for the Combination of Atomic Orbitals
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The linear combination of atomic orbitals to form molecular orbitals takes place only if the following conditions are satisfied: 1. The combining atomic orbitals must have the same or nearly the same energy. 2. The combining atomic orbitals must have the same symmetry about the molecular axis. 3. The combining atomic orbitals must overlap to the maximum extent.
Detailed Explanation
There are specific criteria that must be met for atomic orbitals to combine effectively and form stable molecular orbitals. First, the energy levels of the combining atomic orbitals should be similar; otherwise, they cannot form a stable bond. Second, these orbitals need to have the same spatial orientation (symmetry) for effective overlap, meaning that similar shaped orbitals can interact better. Lastly, to maximize overlap, the orbitals must come as close to each other as possible, which enhances the chances of forming a strong bond.
Examples & Analogies
Think of it like two puzzle pieces. For them to fit together (form a bond), they need to have similar shapes (energy levels) and slots (symmetry) that can interlock. If one piece is from a different puzzle (too high energy), it will never fit. Similarly, if the slots don’t match up, they simply won’t connect properly. The closer they can get (maximum overlap), the better the connection will be.
Key Concepts
-
Molecular Orbitals: Regions in a molecule with a high likelihood of electron presence formed from atomic orbitals.
-
Bonding MOs: A stable molecular orbital formed by in-phase combination of atomic orbitals.
-
Antibonding MOs: A less stable molecular orbital formed by out-of-phase combination of atomic orbitals.
-
Bond Order: Measurement of bond strength determined by the ratio of bonding to antibonding electrons.
Examples & Applications
In the hydrogen molecule (H2), the 1s atomic orbitals combine to form a σ1s bonding molecular orbital and a σ*1s antibonding molecular orbital.
In oxygen (O2), the molecular orbital configuration (σ1s)² (σ1s)² (σ2s)² (σ2s)² (σ2pz)² (π2px)² (π2py)² (π2px)¹ (π2py)¹ indicates two unpaired electrons, rendering O2 paramagnetic.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In phase together they stay, bonding’s the easiest way!
Stories
Imagine two kids on a seesaw, where they have to move together in harmony to stay balanced - just like atomic orbitals combining to form stable molecular orbitals.
Memory Tools
BOA to remember Bonding is Optimal Alignment.
Acronyms
MOLE
Molecular Orbitals Lower Energy for stability.
Flash Cards
Glossary
- Molecular Orbital
A region in a molecule where the probability of finding an electron is relatively high, formed from the combination of atomic orbitals.
- Bonding Molecular Orbital
A molecular orbital that is lower in energy than the atomic orbitals that formed it, promoting stability.
- Antibonding Molecular Orbital
A molecular orbital that is higher in energy than the atomic orbitals that formed it, leading to destabilization.
- Linear Combination of Atomic Orbitals (LCAO)
A method for combining atomic orbitals to form molecular orbitals.
- Bond Order
The difference between the number of bonding and antibonding electrons divided by two, indicating the strength of a bond.
Reference links
Supplementary resources to enhance your learning experience.
