Applications of Surface Tension
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's start our exploration of surface tension. Can anyone tell me what surface tension is?

Is it the force that makes water form droplets?

Exactly! Surface tension is the elastic-like force at the surface of a liquid. It occurs because the molecules at the surface are attracted to each other more than they are to the surrounding air. This is due to cohesive forces. Can anyone give me an example of surface tension in action?

I've seen insects like water striders walk on water without sinking.

Correct! The surface tension allows those insects to walk on water. So remember, surface tension is strong enough to support the weight of insects despite their density being greater than water. We can abbreviate this using the acronym 'CLOSE S', which stands for 'Cohesive Forces Leading to Surface Elasticity'.

That’s a helpful way to remember it!
Effect of Temperature on Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss how temperature plays a role in surface tension. Can any of you explain what happens to surface tension as temperature increases?

I think it decreases, right?

Exactly! As temperature rises, the cohesive forces among molecules weaken, leading to a decrease in surface tension. Can someone explain why that might happen?

Because the molecules move faster and there's less attraction between them?

That's right! Increased kinetic energy allows molecules to overcome cohesive forces. To recap, we can remember the saying, 'As heat climbs, surface tension declines.'
Practical Applications and Examples
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's move on to some practical applications of surface tension. What are some ways we see surface tension in our daily lives?

Soap bubbles! They rely on surface tension to keep their shape.

Definitely! The surface tension must balance the internal pressure in the bubble. Can anyone think of another example?

Water drops on a leaf!

Exactly, water beads up due to high surface tension. Remember the term 'Adhesion vs. Cohesion' to distinguish between how liquids interact with themselves versus with other materials. Lastly, we state, 'Higher tension means tighter drops'.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Surface tension arises from the cohesive and adhesive forces among fluid molecules, significantly affecting both liquid behavior and interactions with solids and gases. This section elucidates its definition, importance in practical applications like raindrops and insect locomotion, and its dependence on temperature.
Detailed
Applications of Surface Tension
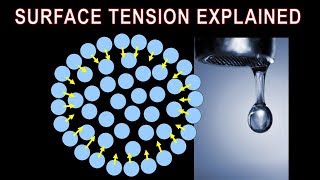
Surface tension is a crucial physical property of liquids that describes the elastic-like force at the surface of a liquid, resulting from intermolecular cohesive properties of liquid molecules. When viewed closely, the molecules at the surface of a liquid experience different forces compared to those in the bulk of the liquid due to a lack of surrounding molecules on one side. This leads to a net inward force, making the surface behave like a stretched elastic membrane.
In practical applications, surface tension can be observed in cases such as raindrops forming spherical shapes and the ability of certain insects to walk on water. The chapter also discusses how surface tension varies with temperature, emphasizing that as temperature increases, cohesive forces weaken, resulting in reduced surface tension. Understanding surface tension is fundamental in fields such as fluid dynamics, chemical engineering, and material science, allowing for innovations in various applications like detergents, emulsions, and coatings.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Surface Tension
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
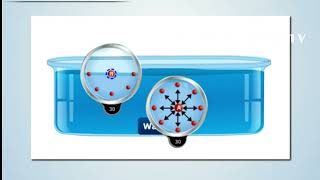
As the name implies that at the interface of the two fluids or fluid with two liquids or fluid and the gas what it actually happens it if you look at it that way that I have the liquid molecules if you look at this figures okay. I have the liquid molecules and this is the free surface I have the gas. So if I look at what is the intermolecular forces is there, okay like for example if fluid is at rest conditions, the molecules at these points is having the attracting force between the surrounding molecules. There is a same molecules are there so the cohesive force are acting on this. So these cohesive force are making these fluid molecules in moment, moment considerations. But when you have the at the surface levels, if you look it that these molecules will have the cohesive force the molecular bounding force between the two like molecules and the adhesive force between the gas molecules and the water molecules.
Detailed Explanation
Surface tension is the phenomenon at the interface between a liquid and gas (or between two liquids) that arises due to the cohesive forces between liquid molecules. At the surface of a liquid, the molecules experience a net inward force because they are surrounded by other liquid molecules below, sides, and above. This creates a 'skin-like' effect that makes the liquid surface behave like an elastic membrane.
Examples & Analogies
Consider a stretched rubber band; it resists any force applied to it, similar to how the surface of water resists forces due to surface tension. This is why small insects, like water striders, can walk on water without sinking.
Surface Tension and Cohesive Forces
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If you imagine it that if these force, the cohesive force is much larger than the adhesive force what is there. That means the molecules which is at the surface, they are going to have a net imbalance force is going to work it. Like you have a surface when which the top of the molecules they are having a pulling effect of the cohesive forces what is working it or bounding forces is acting on that. So this the surface will act like an elastic membranes.
Detailed Explanation
When cohesive forces (forces between similar molecules) exceed adhesive forces (forces between unlike molecules), the surface of a liquid experiences a net inward pull. This imbalance leads to the phenomenon we call surface tension. The analogy used here is helpful: just as a stretched elastic material pulls back when you apply pressure, the surface behaves similarly, creating tension within the liquid surface.
Examples & Analogies
Think about a soap bubble. The film of soap has a layer of water on it, where cohesive forces create a tension that keeps the bubble intact and spherical. Just as a balloon keeps air inside due to its surface tension, liquids shape themselves to minimize surface area.
Surface Tension in Everyday Life
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So that is why small insects can walk on the waters, because of the surface tension forces that what is give the force component because of that small insect can they can walk on the on a water and even if they are having the density is more than that, that is because of the surface tension forces that what resulting surface tension force can result in that ones.
Detailed Explanation
Surface tension is not just a theoretical concept but has tangible applications in nature. It enables certain insects, such as the water strider, to walk on water without sinking. This is possible because the surface tension force is strong enough to support their weight, despite the insects themselves being denser than water.
Examples & Analogies
Imagine trying to balance on a tightrope. The thinner the tightrope, the more careful you need to be. Similarly, water striders balance on water's surface tension, which is the 'tightrope' they walk across.
Surface Tension Measurement
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As a result of these effects, the force what will be resultant per unit length will define as a surface tension forces okay. So this is what it happens it. Because of that if you look at the small insects they can walk on the waters, because of the surface tension forces that what is give the force component because of that small insect can they can walk on the on a water and even if they are having the density is more than that, that is because of the surface tension forces that what resulting surface tension force can result in that ones.
Detailed Explanation
Surface tension can be quantified and is defined as the force per unit length acting along the surface of a liquid. For example, surface tension for water at 20 degrees Celsius is approximately 0.074 N/m. This measurement helps in understanding how different liquids interact with solids.
Examples & Analogies
When you fill a glass with water, you might notice that the water can rise slightly above the rim without spilling. This phenomenon occurs due to surface tension acting at the surface of the water, allowing it to hold its shape temporarily even when the forces exceed the physical boundaries of the glass.
Temperature Effects on Surface Tension
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As the temperature is going to increase it so you will have a decrease of the cohesive forces. As the cohesive forces decreases, you can know if the net force is going to decrease. So resulting effect the surface tensions will have a decreasing trend. So we can when you have a interface between the liquid to liquid or the gas to liquid, you will have a the surface will be act like a elastic membranes with a tension forces.
Detailed Explanation
Temperature plays a significant role in determining surface tension. As the temperature increases, the cohesive forces between liquid molecules tend to weaken, leading to a decrease in surface tension. Therefore, warmer liquids usually have lower surface tension compared to when they are colder.
Examples & Analogies
If you've ever boiled water, you'll notice that the steam rises off the water surface. As the water heats up, the increased thermal energy weakens the cohesive forces between water molecules, resulting in lower surface tension — the water behaves differently when hot compared to when it is cold.
Surface Tension and Pressure Differences
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If you consider the raindrop if you look at that, if you take that drop and make it two splits. At the interface surface there will be surface tension is working it. The surface tension force is acting between the water and the air.
Detailed Explanation
The interface between liquids and gases affects pressure differences within droplets. When you consider a raindrop, the surface tension creates a pressure difference between the inside of the droplet and the surrounding air. Understanding this mechanism allows us to predict the behavior of droplets under different conditions.
Examples & Analogies
Picture a water droplet on a car windshield. The round shape indicates that the surface tension is holding the droplet together, creating a pressure equilibrium between the inside and outside forces. If you tilt the car, the droplet may slide and re-shape, demonstrating the dynamic nature of these forces.
Key Concepts
-
Surface Tension: The cohesive forces at the surface of a liquid create an elastic-like membrane effect.
-
Temperature Dependence: As temperature increases, the cohesive forces weaken, leading to a decrease in surface tension.
-
Practical Examples: Surface tension influences the shape of raindrops, enables insects to walk on water, and plays a role in bubble formation.
Examples & Applications
Raindrops form spherical shapes due to surface tension.
Insects like water striders can walk on water because of surface tension.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When droplets gather, big and round, surface tension's where they are found.
Stories
Once upon a time, a tiny water strider walked across a pond, light as a feather, because the water held tight due to surface tension.
Memory Tools
Remember 'CAP' for understanding surface tension: Cohesion, Adhesion, and Pressure difference.
Acronyms
Use 'S.T.E.P' to remember surface tension
Surface Tension's Effects on Pressure.
Flash Cards
Glossary
- Surface Tension
The elastic-like force at the surface of a liquid caused by the cohesive force between liquid molecules.
- Cohesion
The attraction between molecules of the same substance.
- Adhesion
The attraction between molecules of different substances.
- Pressure Difference
The variation in pressure exerted across the surface of a liquid due to surface tension.
- Contact Angle
The angle formed between the tangent to the liquid surface and the solid surface at the point of contact.
Reference links
Supplementary resources to enhance your learning experience.
