Surface Tension
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are diving into the concept of surface tension. Does anyone know what surface tension is?

Is it the force that makes water droplets round?

Exactly! Surface tension is the force that causes the surface of a liquid to behave like an elastic membrane due to cohesive forces among its molecules. Remember, we can call it the 'membrane effect.'

What happens to surface tension as temperature changes?

Great question! As temperature increases, the cohesive forces decrease, thus reducing surface tension. This relationship shows how temperature affects the molecular interactions in liquids.

Can you give an example of surface tension in action?

One classic example is a water strider, an insect that can walk on water thanks to surface tension!

So it's like a tiny trampoline for them?

Exactly, a great analogy! Surface tension provides the necessary resistance for small creatures to walk on liquid surfaces.

In summary, surface tension is crucial in many natural and practical phenomena, from raindrops to how we see insects moving on water.
Cohesive and Adhesive Forces
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's dig deeper into the forces that create surface tension: cohesive and adhesive forces. Who can explain these terms?

Cohesive forces are the attraction between the same type of molecules, like water with water?

Correct! And adhesive forces are different?

Adhesive forces are between different molecules, like water and air.

Exactly right! The strength of cohesive forces compared to adhesive forces can determine whether a liquid will wet a surface or not.

What does that look like in real life?

Well, when you see water beading up on a leaf, that's cohesive forces at play, while if water spreads on a glass, that's adhesive forces overcoming cohesion.

What happens if cohesion is much stronger?

Then we observe strong surface tension, making it difficult for the liquid to spread, like oil on water.

In summary, the balance of cohesive and adhesive forces is vital for understanding the behavior of liquids on various surfaces.
Applications of Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about how surface tension applies to everyday life. Can anyone name an application?

Soap bubbles?

Exactly! Soap bubbles form due to surface tension of liquid with air. Can anyone explain why they are round?

It's because surface tension minimizes the surface area!

Exactly! This is why bubbles are spherical. They represent an equilibrium state where surface tension is balanced.

Are there other applications?

Absolutely! Consider ink in a fountain pen: surface tension helps the ink to flow smoothly to the tip.

And cleaning wipes work because they break down surface tension, right?

Correct again! By breaking the surface tension of a liquid, cleaning wipes can effectively spread and clean surfaces.

To summarize, surface tension plays a vital role in various practical applications, from bubbles to ink flow, showcasing its importance in life.
Quantifying Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's explore how we can quantify surface tension. Who knows how we measure it?

Is it through experiments with drops?

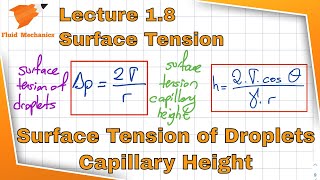
Yes! One method involves measuring the size of a drop that detaches from a surface, using the surface tension formula.

What's the formula?

The formula relates to the force exerted on the length of the surface, usually expressed in Newtons per meter (N/m).

So we can calculate surface tension if we know the force and the circumference of the droplet?

Precisely! This calculation helps us quantify surface tension effectively.

Does this mean different liquids have measurable tensions?

Exactly! Each liquid has a specific surface tension that influences its behavior in various conditions.

Let's wrap up. We learned that measuring surface tension involves experiments and calculations, key to understanding fluid behavior.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Surface tension is the net force acting at the interface of two fluids or a fluid with gas, caused by cohesive and adhesive forces among molecules. It creates an elastic membrane effect, impacting phenomena like raindrops, insect locomotion, and liquid-solid interactions. As temperature increases, surface tension decreases due to reduced cohesive forces among fluid molecules.
Detailed
Surface Tension
Surface tension is defined as the force acting per unit length at the interface between two fluids, such as liquid and gas or liquid and liquid. This phenomenon arises from the cohesive forces among liquid molecules that pull them together, which is stronger for molecules within the bulk of the liquid compared to those at the surface. This leads to an imbalance of forces that creates a membrane-like effect on the surface of the liquid.
Key Concepts
- Cohesive vs. Adhesive Forces: Cohesive forces act between like molecules (e.g., water molecules), while adhesive forces act between different substances (e.g., water and air).
- Imbalance of Forces: Molecules at the surface experience a net inward force due to cohesive forces, making the surface behave like a stretched elastic membrane.
- Effects of Surface Tension: Surface tension allows small insects to walk on water and dictates the behavior of raindrops.
- Temperature Dependency: As temperature increases, cohesive forces decrease, leading to a reduction in surface tension.
Surface tension can also create pressure differences across the liquid interface, as demonstrated in raindrop formation. We can measure surface tension quantitatively, and it acts critically in various real-life applications, such as in wetting liquids and the behavior of bubbles and droplets. Overall, understanding surface tension is essential in fluid mechanics and applications in chemical engineering.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding Surface Tension
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
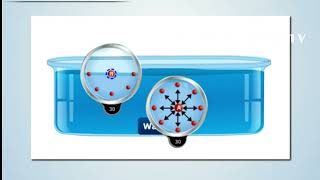
As the name implies that at the interface of the two fluids or fluid with two liquids or fluid and the gas, what it actually happens it if you look at it that way that I have the liquid molecules if you look at this figures okay. I have the liquid molecules and this is the free surface I have the gas. So if I look at what is the intermolecular forces is there, okay like for example if fluid is at rest conditions, the molecules at these points is having the attracting force between the surrounding molecules. There is a same molecules are there so the cohesive force are acting on this.
Detailed Explanation
Surface tension occurs at the interface between two fluids, such as a liquid and a gas. In a liquid at rest, molecules experience cohesive forces from other molecules around them, meaning each molecule pulls on its neighbors. However, at the surface, molecules don’t have neighbors above them (in air, for instance), leading to an imbalance in these forces. This imbalance creates a 'skin' effect on the surface of the fluid, making it behave like an elastic membrane.
Examples & Analogies
Think of surface tension like a trampoline. If you stand in the middle, the fabric stretches down in the center, but you have more support around you. Similarly, molecules at the surface of a liquid experience stronger cohesive forces from the sides and below than from above, creating that tight surface.
Cohesive vs. Adhesive Forces
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
But when you have the at the surface levels, if you look it that these molecules will have the cohesive force the molecular bounding force between the two like molecules and the adhesive force between the gas molecules and the water molecules.
Detailed Explanation
At the surface of a liquid, cohesive forces (the attraction between like molecules, such as water) and adhesive forces (the attraction between different substances, like water and air) operate simultaneously. If cohesive forces are stronger than adhesive forces, the liquid molecules will 'pull' together, enhancing surface tension. This concept is pivotal for understanding how liquids behave with other substances.
Examples & Analogies
Consider how raindrops form on a car's windshield. The cohesive forces within the raindrop are stronger than the adhesive forces between the water and glass, causing the raindrop to maintain its shape instead of spreading out.
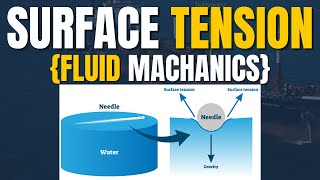
Surface Tension Effect on Insects
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
That means the molecules which is at the surface, they are going to have a net imbalance force is going to work it. Like you have a surface when which the top of the molecules they are having a pulling effect of the cohesive forces what is working it or bounding forces is acting on that. So this the surface will act like an elastic membranes.
Detailed Explanation
Due to surface tension, the liquid surface acts like a stretched elastic membrane. This is strong enough to support small insects, like water striders, which can walk on water despite being denser than the liquid. The surface tension creates a force that counteracts their weight.
Examples & Analogies
Imagine trying to walk on a trampoline. If you place your foot lightly, it can support you because the material is stretched beneath the surface. This is similar to how surface tension allows small insects to move on water without sinking.
Calculating Surface Tension
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So that because of these effects, the force what will be resultant per unit length will define as a surface tension forces okay.
Detailed Explanation
Surface tension can be quantified as a force acting along a unit length (N/m). This measurement is critical in applications ranging from engineering to biological systems, influencing phenomena like capillary action and the formation of droplets.
Examples & Analogies
You can see surface tension in action when you have a straw in a glass of water. If you place your finger over the top of the straw and lift it, the water will stay in the straw momentarily due to the surface tension. This force keeps the water locked in place until you remove your finger.
Surface Tension and Temperature
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As the temperature is going to increase it so you will have a decrease of the cohesive forces.
Detailed Explanation
Temperature has an impact on surface tension; as temperature rises, the energy of the molecules increases, causing the cohesive forces to decrease. Consequently, surface tension tends to decrease as well, making it easier for substances to mix or spread.
Examples & Analogies
Think about how cooking oil behaves when it’s heated. At room temperature, it has a higher surface tension and tends to bead up on a surface. When heated, however, it spreads more easily, indicating a decrease in surface tension.
Key Concepts
-
Cohesive vs. Adhesive Forces: Cohesive forces act between like molecules (e.g., water molecules), while adhesive forces act between different substances (e.g., water and air).
-
Imbalance of Forces: Molecules at the surface experience a net inward force due to cohesive forces, making the surface behave like a stretched elastic membrane.
-
Effects of Surface Tension: Surface tension allows small insects to walk on water and dictates the behavior of raindrops.
-
Temperature Dependency: As temperature increases, cohesive forces decrease, leading to a reduction in surface tension.
-
Surface tension can also create pressure differences across the liquid interface, as demonstrated in raindrop formation. We can measure surface tension quantitatively, and it acts critically in various real-life applications, such as in wetting liquids and the behavior of bubbles and droplets. Overall, understanding surface tension is essential in fluid mechanics and applications in chemical engineering.
Examples & Applications
Water droplets forming beads on a car roof due to high surface tension.
Insects such as water striders walking on water because of the surface tension effect.
Soap bubbles that form spherical shapes to minimize surface area and balance internal and external pressure.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When you see a water bead, surface tension is at speed! Cohesion's the pull, making droplets cool.
Stories
Imagine a tightrope walker, representing a water strider, balancing on the surface of water. The tension keeps them afloat, showing the power of surface tension.
Memory Tools
CATS - Cohesive, Adhesive, Temperature effects, and Surface tension.
Acronyms
TAPS - Temperature, Adhesive forces, Pressure differences, Surface tension.
Flash Cards
Glossary
- Surface Tension
The force per unit length acting at the interface of two fluids, arising from cohesive forces among liquid molecules.
- Cohesive Forces
The intermolecular forces that cause like molecules to attract each other.
- Adhesive Forces
The forces that act between different types of molecules, promoting attraction.
- Pressure Difference
The difference in pressure across a liquid interface due to surface tension.
- Wetting
The ability of a liquid to spread on a solid surface, influenced by the balance of cohesive and adhesive forces.
Reference links
Supplementary resources to enhance your learning experience.
