Surface Tension and Temperature
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Viscosity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will explore viscosity, a crucial property of fluids that describes their resistance to flow. Can anyone tell me what happens to a fluid when it encounters a force?

It resists the flow, right? Like when I try to stir honey, it moves slowly.

Exactly! That's because honey has a high viscosity. Viscosity is affected by temperature, so let’s explore that. As temperature rises, do you think viscosity increases or decreases?

It probably decreases because higher temperatures make things move more, right?

Correct, great observation! Remember, as we heat the fluid, the molecular motion increases which reduces cohesive forces, affecting viscosity.

To help remember, think of 'VIC' - Viscosity Increases with Cooling!
Effects of Temperature on Viscosity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s examine how temperature affects viscosity in liquids versus gases. Can someone explain what happens in liquids?

In liquids, increasing the temperature usually reduces the viscosity.

Exactly, and what about gases?

For gases, it increases, right? Because they move more randomly at higher temperatures.

Very well! Remember the mnemonic: 'Gases Gain Viscosity when Heating', to keep this clear. Let's also discuss the Sutherland correlation for calculating viscosity in gases.
Understanding Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's shift our focus to surface tension. Who can define surface tension for us?

Isn’t it the force that makes the liquid behave like a stretched elastic membrane?

Yes! Well done! This elastic tendency is observed at the interface between liquids and gases. How do you think temperature affects surface tension?

If temperature increases, the cohesive forces between molecules would decrease, right?

Correct! So, as temperature rises, surface tension decreases. A trick to remember this is 'Surface Tension Shrinks as Temperatures Soar!'
Applications of Viscosity and Surface Tension
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Finally, let’s discuss applications. Why is understanding viscosity important in industries?

It helps in processes like the manufacturing of liquids and paints.

Exactly! It's crucial for optimizing operations. How about surface tension?

Like, small insects walking on water or even in detergents that help water spread better.

Great examples! To summarize, viscosity and surface tension are vital properties in fluid dynamics with numerous applications in nature and technology.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
It provides an overview of the basic principles of viscosity and surface tension, explains how temperature affects these properties in liquids and gases, and highlights the significance of these behaviors in fluid mechanics.
Detailed
Detailed Summary on Surface Tension and Temperature
In this section, we delve into fluid dynamics, specifically focusing on the concepts of viscosity and surface tension. Viscosity is defined as the measure of a fluid's resistance to flow, which is important in understanding how fluids behave under shear stress. The relationship between shear strain rate and shear stress is emphasized, often typified by Newton's law of viscosity. This law states that for Newtonian fluids, the shear stress is directly proportional to the velocity gradient.
Temperature plays a critical role in determining the coefficient of viscosity. As temperature increases, especially in liquids, the cohesive forces between molecules weaken, leading to a decrease in viscosity. Conversely, for gases, the increase in temperature enhances molecular motion, leading to an increase in viscosity. The effects of temperature on viscosity are explored using the Sutherland correlation for different gases and liquids, which provides specific mathematical relationships.
Surface tension, another key concept, refers to the elastic tendency of a fluid surface that makes it acquire the least surface area possible. This is particularly noticeable in the behavior of liquids in contact with gases or other liquids. The underlying forces at play include cohesive forces among similar molecules and adhesive forces between different substances. The temperature's impact on surface tension is discussed, showing that increased temperatures lead to a decrease in surface tension as well, due to weaker intermolecular forces.
Understanding these principles is essential in various engineering and scientific fields, particularly in chemical and fluid engineering applications.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Surface Tension
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As the name implies that at the interface of the two fluids or fluid with two liquids or fluid and the gas what it actually happens it if you look at it that way that I have the liquid molecules if you look at this figures okay. I have the liquid molecules and this is the free surface I have the gas. So if I look at what is the intermolecular forces is there, okay like for example if fluid is at rest conditions, the molecules at these points is having the attracting force between the surrounding molecules. There is a same molecules are there so the cohesive force are acting on this. So this cohesive force are making these fluid molecules in moment, moment considerations.
Detailed Explanation
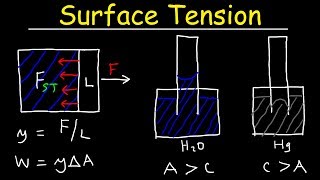
Surface tension occurs at the interface between two different fluids, such as a liquid and gas. At the surface of the liquid, molecules experience forces from all directions except upward, where they encounter air. This results in a net inward force, creating a tension at the surface, which acts like a stretched elastic membrane. These forces can affect the behavior of objects on the surface, like allowing small insects to walk on water.
Examples & Analogies
Imagine a trampoline—when you stand in the middle, the surface stretches downward due to your weight. Just like the trampoline, the surface of water stretches because of the cohesive forces between water molecules, creating surface tension.
Effects of Temperature on Surface Tension
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As the surface tensions is defined is a force acting per unit length and that is what will be N/m, and the at the surface tension the water with air interference 0.074 N/m at 20oC. And if you look at the molecular perspective view that as the temperature is going to increase it so you will have a decrease of the cohesive forces. As the cohesive forces decreases, you can know if the net force is going to decrease. So resulting effect the surface tensions will have a decreasing trend.
Detailed Explanation
Surface tension is quantified as the force exerted along a line at the liquid's surface, measured in newtons per meter (N/m). For example, water has a surface tension of 0.074 N/m at 20°C. However, as temperature rises, the cohesive forces between liquid molecules weaken, which in turn decreases the surface tension. This means that as liquids warm up, they become less 'sticky' at their surfaces.
Examples & Analogies
Think of a warm bubble bath: as the water heats up, the bubbles can become bigger and more easily pop because the surface tension becomes less strong. Warm water allows for more movement and less 'stickiness' of water molecules, making bubbles less stable.
Pressure Difference Due to Surface Tension
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If you have the surface tension if you can now there will be a net balancing effect of the force component which results us the pressure difference. Let us consider it is the surface tension is given very simple examples of raindrops okay. Any raindrop if you look at that, if you take that drop and make it two splits. At the interface surface there will be surface tension is working it. The surface tension force is acting between the water and the air.
Detailed Explanation
Surface tension can create a pressure difference across the surface of a liquid drop. For example, consider a tiny raindrop; the surface tension exerts an inward force that results in higher pressure inside the droplet compared to the outside atmosphere. This pressure difference is fundamental to the droplet's formation and stability.
Examples & Analogies
Think of a balloon: when you blow air into it, the inflating pressure inside the balloon is higher than the pressure outside. Similarly, in a water droplet, the higher internal pressure provides stability, while the surface tension keeps the droplet intact.
Wetting and Contact Angles
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Next interesting point is coming is that if a liquid interfacing with the solids whether it will be wet or not wet, the wetting condition or not wetting condition. That what is a interface between the three of things.
Detailed Explanation
When a liquid meets a solid, its ability to wet the surface depends on the balance of cohesive forces within the liquid and adhesive forces between the liquid and solid. This results in a contact angle: if the angle is less than 90 degrees, the liquid wets the surface; if greater, it does not. This concept is critical in various applications like paint adhesion or inkjet printing.
Examples & Analogies
Consider how water beads up on a waxed car versus how it spreads out on a sponge. This difference in behavior is due to the contact angle—the smooth surface of the wax creates a high contact angle (causing beads), while the porous sponge allows for lower angles (allowing spreading).
Key Concepts
-
Viscosity: The resistance to flow in fluids, critical for understanding fluid dynamics.
-
Surface Tension: The tendency of a liquid's surface to resist external forces.
-
Temperature Effects: Temperature inversely affects viscosity in liquids and directly affects viscosity in gases.
Examples & Applications
Honey flows slowly due to its higher viscosity compared to water.
Small insects can walk on the surface of water due to surface tension.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In heat, some fluids glide, viscous flows with changing tide.
Stories
Imagine a honey bee warming up honey, making it easier to flow, just as heat makes honey liberate its sticky hold.
Memory Tools
VIC - Viscosity Increases with Cooling, remember viscosity and temperature!
Acronyms
CATS - Cohesive And Tensile Surfaces, to remember the forces at play in surface tension.
Flash Cards
Glossary
- Viscosity
A measure of a fluid's resistance to flow.
- Surface Tension
The elastic tendency of a fluid surface that makes it acquire the least surface area possible.
- Shear Stress
The force per unit area exerted parallel to the surface.
- Velocity Gradient
The rate of change of velocity at which fluid elements move.
- Cohesive Force
The intermolecular forces that hold similar molecules together.
- Adhesive Force
The attractive forces between different types of molecules.
Reference links
Supplementary resources to enhance your learning experience.
