Calcination
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Calcination
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’re delving into the process of calcination in cement manufacturing. Can anyone tell me what calcination involves?

I think it’s when limestone is heated?

Exactly! When limestone is heated, it undergoes a chemical process. Does anyone know what happens to the calcium carbonate in limestone during this heating?

It decomposes into calcium oxide and carbon dioxide!

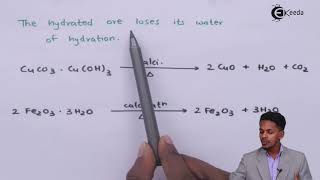
Exactly right! The equation for that reaction is CaCO₃ → CaO + CO₂. Remember, CO₂ is released in gas form which is essential to understand. This process occurs at about 900°C.

So, what is the significance of calcium oxide in cement?

Great question! Calcium oxide is crucial because it combines with other materials in the kiln to form clinker. This means calcination is directly linked to the quality of the final cement product. Always keep in mind, 'Calcium Carbonate Can Only Create' CO₂ and CaO during calcination.

What happens if the limestone is not fully calcined?

If limestone isn’t fully calcined, the quality of cement may suffer because not enough calcium oxide is produced. Therefore, it’s essential to control the heating process carefully.

To wrap up, remember that calcination is where calcium carbonate transforms into vital components for producing cement. Any questions before we move on?
The Chemical Reaction in Calcination
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s focus on the chemical reaction during calcination. Can anyone repeat the formula we discussed?

It’s CaCO₃ transforms into CaO and CO₂!

Correct! That transformation is the essence of calcination. Why do you think this step is important in the context of cement manufacturing?

Because without it, we wouldn’t have calcium oxide, which is needed for the clinker?

Exactly! So remember: 'Calcination Creates Calcium Added to Clinker.' Can you see how every phase is intertwined in the process?

What happens to the carbon dioxide?

Good question! The CO₂ escapes into the atmosphere, which is also a point of concern for environmental emissions from cement manufacturing.

So, reducing emissions is also related to how we manage calcination?

Exactly — effective calcination can lower emissions, making it crucial for both efficiency and environmental impact. Remember: 'Calcium and Carbon balance must be optimal.'
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
During calcination, which occurs at around 900°C, calcium carbonate is transformed into calcium oxide and carbon dioxide, playing a crucial role in the overall cement manufacturing process. Understanding this phase is vital for appreciating how raw materials evolve into usable cement.
Detailed
Detailed Summary of Calcination
Calcination is a pivotal step in the dry process of cement manufacturing, specifically occurring at approximately 900°C. In this stage, calcium carbonate (commonly sourced from limestone) undergoes thermal decomposition to yield calcium oxide (also known as quicklime) and carbon dioxide gas (CO₂). The reaction can be summarized as:
$$\text{CaCO}_3 \rightarrow \text{CaO} + \text{CO}_2 \uparrow$$
This transformation is critical as the produced calcium oxide serves as a key component in the formation of clinker in the subsequent phases of cement production. Proper management of this step ensures the quality and efficiency of the entire manufacturing process, as any loss of carbon dioxide or incomplete calcination can lead to poor cement quality.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Calcination Process
Chapter 1 of 1
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At around 900°C, calcium carbonate (CaCO₃) decomposes to calcium oxide (CaO) and carbon dioxide (CO₂).
CaCO →CaO+CO ↑
3 2
Detailed Explanation
During the calcination process, which occurs around 900°C, calcium carbonate (CaCO₃), found primarily in limestone, is heated. This heat causes the calcium carbonate to break down into two different substances: calcium oxide (CaO) and carbon dioxide (CO₂). This reaction can be illustrated as:
CaCO₃ → CaO + CO₂↑
In this equation, the upward arrow indicates that carbon dioxide gas is released into the atmosphere. The formation of calcium oxide is crucial because it is a key ingredient in the production of cement, forming the basis for further chemical reactions that create the final product.
Examples & Analogies
To think of calcination in real life, consider how baking transforms ingredients. Just like heating dough in an oven causes it to rise and change structure into bread, heating calcium carbonate alters its chemical structure to produce calcium oxide and releases gas. This transformation is essential for cement production and is similar to how the right conditions can lead to new desired properties in cooking.
Key Concepts
-
Calcination: The thermal decomposition of calcium carbonate into calcium oxide and carbon dioxide.
-
Temperature: Calcination occurs at approximately 900°C.
-
Significance: Calcium oxide produced is essential for clinker formation in cement.
Examples & Applications
An example of calcination is the heating of limestone in a kiln, where it decomposes to form lime and gas.
In an industrial cement plant, controlling the calcination temperature is crucial to ensure that CO₂ is effectively released while maximizing CaO production.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When limestone heats up at nine hundred, it splits with flair, CO₂ and CaO declared!
Stories
Once in a hot kiln, a rock named limestone decided to become more than just a stone. It was heated and transformed into a powerful builder called calcium oxide, freeing gas into the air.
Memory Tools
CaCO₃ turns into CaO and CO₂ — 'Calcination Creates Calcium and CO₂.'
Acronyms
C.C.C. - 'Calcination Creates Calcium.'
Flash Cards
Glossary
- Calcination
The thermal decomposition process of calcium carbonate into calcium oxide and carbon dioxide.
- Calcium Carbonate
A chemical compound (CaCO₃) commonly found in limestone, crucial for cement manufacturing.
- Calcium Oxide
A key product of calcination, essential for forming clinker in cement.
Reference links
Supplementary resources to enhance your learning experience.
