Applications Of Equilibrium Constants
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Equilibrium Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're diving into equilibrium constants. Who can tell me what Kc represents in a chemical reaction?

Kc is the equilibrium constant for concentrations of reactants and products.

Exactly! Kc = [products]⁺ / [reactants]⁻, where the brackets indicate concentrations at equilibrium. Why do we use Kp when dealing with gases?

Because Kp uses partial pressures instead of concentrations.

Great! So we can switch between Kc and Kp based on whether we're looking at concentrations or pressures. Remember the formula: Kp = Kc (RT)ⁿ, where n is the change in the number of moles of gas.

Can you give us an example of where we use Kp?

Sure! If we look at the reaction of N2 and O2 producing NO at high temperature, Kp becomes crucial. Can anyone tell me how changes in pressure affect equilibrium?

Le Chatelier’s principle states that if we increase pressure, the equilibrium will shift to the side with fewer gas moles.

Exactly! Remember this principle, it’s key in reaction optimization. Summarizing, Kc is for concentrations, Kp is for partial pressures, and pressures affect equilibria based on the number of gas moles.
Le Chatelier's Principle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's discuss Le Chatelier's principle in more detail. What happens if we add more reactants to our reaction system?

The system will shift to the right to use up those extra reactants.

Correct, and what if we removed a product? How does the equilibrium respond to that?

Then the equilibrium shifts to the right again to produce more of that product.

Exactly! It’s all about restoring balance. Changes in temperature are a bit different though; how do they affect exothermic versus endothermic reactions?

For exothermic reactions, increasing temperature shifts equilibrium left, while for endothermic, it shifts right.

Well done! This principle is essential in industrial applications. For example, we manipulate conditions to maximize yield for ammonia synthesis in the Haber process. Summarizing, Le Chatelier’s principle helps predict the direction of shifts in equilibrium based on changes to concentration, pressure, and temperature.
Applications in Industry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about how these concepts are applied in real-world industrial processes, like the production of ammonia. Why is understanding the equilibrium constant so crucial here?

Because we want the reaction to favor the production of NH3?

Exactly! By optimizing temperature and pressure, we can increase Kc and maximize the yield. What else can you think of that might influence this reaction in a negative way?

A decrease in temperature could slow the reaction down.

Good point! But remember, while lowering temperature can favor the formation of products for exothermic reactions, it also lowers reaction rates. Hence, we balance conditions carefully. Can anyone think of other applications of this knowledge?

In pharmaceuticals, optimizing drug synthesis and stability of products is important too.

Right again! Each application emphasizes the significance of understanding equilibrium. Today we reviewed how equilibrium constants inform our choices to enhance production efficiency.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explains how equilibrium constants (Kc and Kp) can be used to analyze chemical reactions, predict their progress, and understand how changes in concentration, pressure, and temperature affect equilibria. It also explores the applications of these concepts in chemical synthesis and industrial processes.
Detailed
Applications of Equilibrium Constants
Chemical equilibria are essential for understanding various biological and environmental reactions, as well as industrial processes. This section emphasizes how the equilibrium constant, denoted as Kc for concentrations and Kp for partial pressures, plays a crucial role in determining the extent and direction of chemical reactions.
Key Points:
- Equilibrium Constant Definitions: Kc is defined for reactions at a given temperature where the ratio of products to reactants remains constant. Kp is used when dealing with gases and their partial pressures.
- Factors Influencing Equilibrium: Changes in concentration, pressure, and temperature can shift the equilibrium state. According to Le Chatelier’s principle, a system at equilibrium will respond to counteract any change.
- Relevance in Chemical Processes: Understanding K values assist in optimizing conditions for reactions such as ammonia synthesis or combustion processes, improving yields while minimizing costs and energy usage.
- Applications in Real Life: Knowledge of equilibrium allows scientists and engineers to manipulate reactions in various fields—including pharmaceuticals, agriculture, and environmental science—to achieve desired outcomes effectively.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Predicting the Extent of a Reaction
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
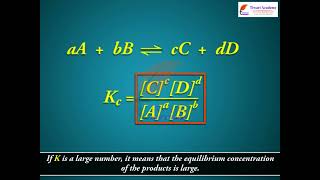
The numerical value of the equilibrium constant for a reaction indicates the extent of the reaction. But it is important to note that an equilibrium constant does not give any information about the rate at which the equilibrium is reached. The magnitude of Kc or Kp is directly proportional to the concentrations of products (as these appear in the numerator of the equilibrium constant expression) and inversely proportional to the concentrations of the reactants (these appear in the denominator). This implies that a high value of K is suggestive of a high concentration of products and vice-versa.
We can make the following generalisations concerning the composition of equilibrium mixtures:
- If Kc > 103, products predominate over reactants, i.e., if Kc is very large, the reaction proceeds nearly to completion... (examples follow)
Detailed Explanation
The equilibrium constant (K) of a reaction shows how far the reaction will go towards products or reactants when it reaches equilibrium. A high K value (greater than 103) suggests that the reaction essentially completes and produces mostly products, while a very low K value (less than 10^-3) indicates that the reactants are favored and the reaction hardly goes forward. It's not about how quickly the reaction occurs; rather, it describes the point at which the system stabilises with certain proportions of products and reactants.
Examples & Analogies
Think of making a cake. If you follow the recipe closely (like maintaining the right ingredients), the cake will turn out well (products formed) and many will enjoy it. However, if you mix too much flour (reactants), you'll likely have something inedible (favoring reactants). The extent of how well the cake turns out (equilibrium constant) reflects how closely you followed the recipe.
Predicting the Direction of the Reaction
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The equilibrium constant helps in predicting the direction in which a given reaction will proceed at any stage. For this purpose, we calculate the reaction quotient Q. The reaction quotient, Q (Qc with molar concentrations and QP with partial pressures) is defined in the same way as the equilibrium constant Kc except that the concentrations in Qc are not necessarily equilibrium values.
For a general reaction: a A + b B c C + d D
Qc = [C]c[D]d / [A]a[B]b
Then,
If Qc > Kc, the reaction will proceed in the direction of reactants (reverse reaction).
If Qc < Kc, the reaction will proceed in the direction of the products (forward reaction).
Detailed Explanation
The reaction quotient (Q) measures the current ratio of concentrations (or pressures) of products to reactants at any point during a reaction. By comparing Q to K (the equilibrium constant), we can predict how the reaction will shift. If Q is greater than K, it indicates there are too many products, leading to a shift towards reactants to restore balance. Conversely, if Q is less than K, more products will be formed until the equilibrium is reached.
Examples & Analogies
Imagine a busy store where products are flying off the shelves. If more products are sold than delivered (Q > K), the store needs to restock (shift towards reactants) to meet customer demand. If too many products accumulate (Q < K), the store probably needs to encourage sales to clear out extra stock (shift towards products).
Applications of Equilibrium Constants in Chemical Processes
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The equilibrium constant expressions can be used to calculate equilibrium concentrations, thus demonstrating their significance in various chemical processes. Changes in conditions, such as temperature, concentration, or pressure, will shift the position of equilibrium and affect Kc or Kp. For example, if the temperature rises for an exothermic reaction (releases heat), it will decrease the value of Kc as the equilibrium shifts to favor reactants.
Detailed Explanation
Equilibrium constants are crucial in understanding how reactions behave under different conditions. For example, if you heat a reaction that typically releases heat (an exothermic reaction), the added heat will push the reaction to favor the reactant side, thus reducing the value of the equilibrium constant Kc. Similarly, increasing the concentration of a reactant may drive the reaction toward products, making them more favorable. Therefore, Kc or Kp helps predict how much product will form under set conditions.
Examples & Analogies
Consider a party where guests (reactants) can either mingle (react) to form groups (products) or hang around alone (remain as reactants). If you invite more guests (increased reactants), the likelihood of forming groups increases. However, if you crank up the heat (raise temperature), it might scatter the guests, making them less likely to form groups. This analogy mirrors how changing conditions impact chemical equilibria.
Importance of Temperature in Equilibrium
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Whenever an equilibrium is disturbed by a change in the concentration, pressure or volume, the composition of the equilibrium mixture changes because the reaction quotient, Qc no longer equals the equilibrium constant, Kc. However, when a change in temperature occurs, the value of equilibrium constant, Kc is changed. In general, the temperature dependence of the equilibrium constant depends on the sign of ΔH for the reaction.
Detailed Explanation
Temperature changes can drastically affect equilibrium constants. For endothermic reactions (those that absorb heat), increasing the temperature raises K and favors products, while decreasing temperature does the opposite. Conversely, for exothermic reactions (those that release heat), increasing temperature lowers K and favors reactants, while a decrease would shift the equilibrium towards products. Understanding this relationship allows chemists to manipulate reactions to favor desired outcomes.
Examples & Analogies
Imagine brewing coffee. If you heat water (higher temperature), the coffee grounds (reactants) extract flavor (products) quicker, enhancing the brew. However, if you let it cool (lower temperature), extraction slows, and you might lose the coffee's flavor. Adjusting temperature similarly influences chemical reactions, guiding outcomes and yields.
Key Concepts
-
Equilibrium Constants: Kc and Kp for measuring equilibria.
-
Le Chatelier's Principle: Response of equilibrium to changes in conditions.
-
Dynamic Equilibrium: Continuous process with no net change in concentration.
Examples & Applications
In the synthesis of ammonia, manipulating pressure and temperature influences the equilibrium constant K.
For the reaction N2 + 3H2 ⇌ 2NH3, Kc changes with temperature, showcasing the application's significance in industrial chemistry.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Kc and Kp, the pressure and concentration, Keep equilibrium in perfect relation.
Stories
Imagine a seesaw at play, when you add weight to one side, the other gives way; just like reactions in a chemical way.
Memory Tools
Kc and Kp: Keep Calm, keep Pressure right.
Acronyms
Kc = Concentration's Kings - watch how they balance.
Flash Cards
Glossary
- Equilibrium Constant (Kc)
A value that expresses the ratio of product concentrations to reactant concentrations at equilibrium.
- Equilibrium Constant (Kp)
Similar to Kc but expressed in terms of partial pressures for gaseous equilibria.
- Le Chatelier's Principle
A principle stating that if a change is applied to a system at equilibrium, the equilibrium will shift to counteract that change.
- Dynamic Equilibrium
A state of balance in which the rates of forward and reverse reactions are equal.
Reference links
Supplementary resources to enhance your learning experience.
