Ionization of Adids and Bases
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ionization
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

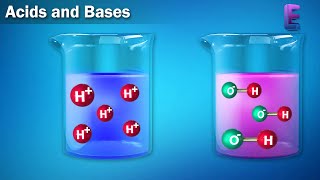
Good morning, everyone! Today, we will discuss the ionization of acids and bases. Can anyone remind us what an acid is?

An acid is a substance that donates hydrogen ions, right?

Exactly! And a strong acid, like hydrochloric acid, dissociates completely in water to produce a high concentration of hydronium ions. This is opposed to weak acids, which only partially dissociate.

So, a weak acid will have an equilibrium of both ions and undissociated molecules?

Right again! This leads us to the concept of ionization constants. Can anyone tell me what Ka represents?

Ka is the ionization constant for acids, showing how well an acid dissociates.

Perfect! Remember, stronger acids have higher Ka values. Let's keep this in mind as we move through the session.
Comparing Strong and Weak Electrolytes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, onto the differences between strong and weak electrolytes. Who can give me an example of a strong base?

NaOH, sodium hydroxide, is a strong base.

Exactly! And it fully dissociates in water to give hydroxide ions. Let's contrast this with a weak base, like ammonia. What happens when ammonia is dissolved in water?

It partially ionizes, establishing an equilibrium between the ions and the undissociated molecules.

Correct! The equilibrium involving ammonia can be represented as NH3 + H2O ⇌ NH4+ + OH-.

And for weak acids like acetic acid, we have a similar situation.

Exactly! The key takeaway is that equilibrium is established for weak acids and bases, allowing us to measure their ionization through their respective Ka and Kb values.
Understanding pH and Ionization Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's shift our focus to pH, which is a crucial aspect of our discussion. Remember that pH is related to the concentration of H+ ions in a solution. What is the formula for calculating pH?

pH = -log[H+].

Great! Now, if we know the Ka value of an acid, how can we infer its strength?

Higher Ka means a stronger acid because it indicates greater ionization.

Exactly. And similarly, Kb does the same for bases. Can anyone tell me the relationship among Ka, Kb, and Kw?

Ka × Kb = Kw, right?

Yes! This relationship is fundamental in understanding the behavior of conjugate acid-base pairs.

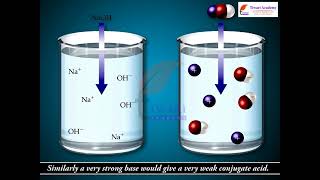
So, strong acids have weak conjugate bases, and weak acids have stronger conjugate bases?

Exactly! As we conclude this session, remember that pH, Ka, and Kb are all interconnected in describing acid-base behavior.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section details how strong acids and bases are characterized by their ability to completely dissociate in water, leading to the generation of H3O+ ions and OH- ions respectively. Contrarily, weak acids and bases are partially ionized, establishing an equilibrium between the undissociated species and the ions produced. The concepts of ionization constants (Ka for acids and Kb for bases) are also introduced.
Detailed
Ionization of Acids and Bases
Acids and bases are crucial components in various chemical equilibriums that occur in aqueous solutions. When discussing their ionization, acids are substances that donate protons (H+) and bases are those that accept protons. Strong acids, such as HCl and H2SO4, dissociate almost completely in solution, producing a higher concentration of H3O+ ions. Conversely, weak acids like acetic acid (CH3COOH) only partially dissociate, creating a mixture of dissociated ions and undissociated molecules at equilibrium.
The degree of ionization indicates the strength of an acid or base; higher ionization corresponds to more potent acids or bases. This section provides insight into the concept of ionization constants (Ka and Kb), which quantify the strength of weak acids and bases respectively. Additionally, it introduces the ion product of water (Kw) and its implications in determining the pH of solutions. By understanding the paradigm of strong and weak electrolytes, students can gain insights into the behavior of acids and bases in various chemical reactions and biological systems.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Strong Acids and Bases
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Arrhenius concept of acids and bases becomes useful in case of ionization of acids and bases as mostly ionizations in chemical and biological systems occur in aqueous medium. Strong acids like perchloric acid (HClO4), hydrochloric acid (HCl), hydrobromic acid (HBr), hydriodic acid (HI), nitric acid (HNO3) and sulphuric acid (H2SO4) are termed strong because they are almost completely dissociated into their constituent ions in an aqueous medium, thereby acting as proton (H+) donors. Similarly, strong bases like lithium hydroxide (LiOH), sodium hydroxide (NaOH), potassium hydroxide (KOH), caesium hydroxide (CsOH) and barium hydroxide Ba(OH)2 are almost completely dissociated into ions in an aqueous medium giving hydroxyl ions, OH–. According to Arrhenius concept they are strong acids and bases as they are able to completely dissociate and produce H3O+ and OH– ions respectively in the medium.
Detailed Explanation
This chunk discusses the behavior of strong acids and bases according to the Arrhenius theory. It explains that strong acids dissociate completely in water to release hydrogen ions (H+), making them effective proton donors. Examples of strong acids include HCl and H2SO4. Strong bases, on the other hand, dissociate to release hydroxyl ions (OH–), with examples like NaOH and KOH. The complete dissociation in aqueous solutions is a key feature that makes them classified as strong.
Examples & Analogies
Think of strong acids like HCl as fully opened faucets, where water (which represents H+) flows out continuously without any restriction. Similarly, strong bases like NaOH are like fully opened drainpipes, allowing OH– to flow out freely. In both cases, there’s no blockage, allowing for complete movement, much like how these substances completely ionize in solutions.
Weak Acids and Ionization Equilibrium
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consider the acid-base dissociation equilibrium of a weak acid HA, HA(aq) + H2O(l) ⇌ H3O+(aq) + A–(aq). In section 6.10.2 we saw that acid (or base) dissociation equilibrium is dynamic involving a transfer of proton in forward and reverse directions. Now, the question arises that if the equilibrium is dynamic then with passage of time which direction is favoured? What is the driving force behind it? In order to answer these questions we shall deal with the issue of comparing the strengths of the two acids (or bases) involved in the dissociation equilibrium.
Detailed Explanation
This part dives into weak acids, highlighting that they do not completely dissociate in water, unlike strong acids. In the example provided, a weak acid HA establishes an equilibrium in water, producing hydronium ions (H3O+) and conjugate base ions (A–). The equilibrium is dynamic; it can shift depending on the strengths of the acids in play. If HA is a stronger acid compared to H3O+, it will primarily dissociate, which affects the concentrations of products and reactants over time.
Examples & Analogies
Imagine a weak battery that can only provide a limited amount of power (representing a weak acid). As time passes, if there's a strong demand for power (like a strong acid), the battery won't be able to fulfill that need completely. Instead, it will often switch between providing power and resting, similar to how weak acids set up an equilibrium between their dissociated and undissociated forms.
Ionization Constants and Their Implications
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Typical weak acids are nitrous acid (HNO2), hydrofluoric acid (HF), and acetic acid (CH3COOH). It should be noted that the weak acids have very strong conjugate bases. For example, NH2–, O2– and H– are very good proton acceptors and thus, much stronger bases than H2O.
Detailed Explanation
This chunk emphasizes the nature of weak acids and their associated ionization constants (Ka). Weak acids do not fully ionize in solution, which results in the presence of their conjugate bases that are comparably stronger. The stronger the conjugate base, the better it is at accepting protons. This dynamic plays an important role in determining the behavior of these acids in various chemical reactions.
Examples & Analogies
Consider the idea of a light dimmer switch. A weak acid is like having the dimmer set to a low level where the light (representing H+) is not very bright; it can brighten up (ionize more) if there is a strong ‘demand’ for light (reactivity). Meanwhile, the dimmer also produces a 'shadow' which reflects further stronger bases, indicating that the base (conjugate) can respond well in its own manner when more light (H+) is required.
Water's Role in Acid-Base Reactions
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Some substances like water are unique in their ability of acting both as an acid and a base. We have seen this in case of water in section 6.10.2. In presence of an acid, HA it accepts a proton and acts as the base while in presence of a base, B– it acts as an acid by donating a proton.
Detailed Explanation
This chunk introduces the unique properties of water as an amphoteric substance, meaning it can act both as an acid and a base. When in contact with an acid, it behaves as a base (accepting a hydrogen ion). When it interacts with a base, it behaves as an acid (donating a hydrogen ion). This dual role is significant for various chemical reactions and biological processes.
Examples & Analogies
Water can be likened to a versatile player in a sport who can be both offensive and defensive. Depending on the game scenario (acid/base), it can either absorb pressure (accept a proton) or release it (donate a proton), showcasing its adaptability in maintaining equilibrium.
The pH Scale and Its Relevance
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The pH scale (pH = –log[H+]) for the hydrogen ion concentration has been introduced. The pH of pure water is given as: pH = –log(10–7) = 7. Acidic solutions possess a concentration of hydrogen ions, [H+] > 10–7 M, while basic solutions possess a concentration of hydrogen ions, [H+] < 10–7 M.
Detailed Explanation
This section outlines the pH scale, which is crucial for expressing the acidity or basicity of solutions. The scale is logarithmic and indicates how acidic or basic a solution is based on the concentration of hydrogen ions (H+). A pH of 7 represents neutrality, with lower values indicating acidity and higher values indicating basicity.
Examples & Analogies
You can think of pH as a scale for sweetness or bitterness in food. Just like how a perfect recipe balances flavors, in chemistry, a balanced pH level indicates the ideal acidity for various substances. Pure water, with its neutral pH of 7, is like a perfectly seasoned dish—neither too sweet (acidic) nor too bitter (basic).
Key Concepts
-
Ionization: The process where acids or bases dissociate into ions in solution.
-
Strong acids/bases: Substances that fully dissociate in solution.
-
Weak acids/bases: Substances that only partially dissociate, establishing an equilibrium.
-
Ionization constants (Ka and Kb): Quantify the extent of ionization for weak acids and bases.
-
pH: A logarithmic scale used to specify the acidity or basicity of a solution.
Examples & Applications
Hydrochloric acid (HCl) is a strong acid that dissociates completely into H+ and Cl- ions in water.
Acetic acid (CH3COOH) is a weak acid that only partially dissociates in solution, establishing an equilibrium.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Acids and bases, they react with ease,
Stories
Imagine a party where strong acids invite everyone to dissociate completely, while weak acids stay reserved and interact only partially.
Memory Tools
To remember 'Ka' for acids and 'Kb' for bases:
Acronyms
Use 'A.C.I.D.' to remember
Acid Donates
Ionizes
Dissociates.
Flash Cards
Glossary
- Ionization
The process by which an atom or molecule gains or loses electrons to form ions.
- Ka
The ionization constant for weak acids; indicates the strength of the acid.
- Kb
The ionization constant for weak bases; indicates the strength of the base.
- Kw
The ion product of water; it is the product of the concentrations of hydrogen ions and hydroxide ions in water.
- pH
A measure of the acidity or basicity of a solution, calculated as the negative logarithm of the hydrogen ion concentration.
Reference links
Supplementary resources to enhance your learning experience.
