Relation Between Ka and Kb
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ka and Kb
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to explore the relationship between the acid dissociation constant, or Ka, and the base dissociation constant, Kb. Can anyone tell me what an acid dissociation constant represents?

Isn't it a measure of how easily an acid donates a proton?

Correct! The higher the Ka value, the stronger the acid. Now, what about Kb?

Kb measures how readily a base accepts a proton.

Exactly! Both constants give us an idea about the strength of the acid or base in solution.
Relation between Ka and Kb
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's talk about the relationship between Ka and Kb. For conjugate acid-base pairs, we find that Ka multiplied by Kb equals Kw. Who can tell me what Kw is?

Kw is the ion product of water!

Correct! It's always 1.0 × 10^−14 at 25°C. So, if we have a strong acid with a high Ka value, what can we infer about its conjugate base?

It would have a low Kb value, meaning it's a weak base!

Absolutely! This understanding allows us to derive one constant from the other, which is very useful in predicting the behavior of acids and bases in various conditions.
Practical Application
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's apply this knowledge. If we know that Ka for NH4+ is 5.6 × 10−10, what is Kb for NH3?

We can use the equation Kw = Ka × Kb to find it!

Exactly! So, Kb would be Kw divided by Ka, or 1.0 × 10^−14 / 5.6 × 10−10.

That gives us Kb = 1.8 × 10−5!

Great job! This example shows why knowing the constants helps us in chemistry.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section elaborates on how the strengths of acids and bases are quantitatively related through their dissociation constants (Ka and Kb). It introduces the concept that for any conjugate acid-base pair, the product of the two constants equals the ion product of water (Kw). Thus, knowing one of the constants allows for the calculation of the other.
Detailed
In the context of acid-base equilibria, the section explores the relationship between the acid dissociation constant (Ka) and the base dissociation constant (Kb) of conjugate acid-base pairs. For example, when examining ammonium (NH4+) as a conjugate acid of ammonia (NH3), the dissociation reactions can be represented:
-
NH4+(aq) + H2O(l) ⇌ H3O+(aq) + NH3(aq)
Here, Ka is introduced, representing the strength of NH4+ as an acid, calculated from the concentrations at equilibrium. -
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH−(aq)
Kb is introduced for ammonia, indicating its strength as a base.
By deriving the relationship from both equations, it is seen that: Ka × Kb = Kw, where Kw = 1.0 × 10^−14 at 25°C. This illustrates that strong acids have weak conjugate bases and vice versa. The section emphasizes that understanding the relationship allows for the derivation of the unknown constant if one is known, providing insight into the equilibrium behaviors of acids and bases.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Ka and Kb Relationship
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
As seen earlier in this chapter, Ka and Kb represent the strength of an acid and a base, respectively. In case of a conjugate acid-base pair, they are related in a simple manner so that if one is known, the other can be deduced.
Detailed Explanation
Ka (the acid dissociation constant) measures how completely an acid donates its protons to water, while Kb (the base dissociation constant) measures how completely a base accepts protons from water. For any conjugate acid-base pair, if we know the value of Ka for the acid, we can find Kb for its conjugate base using the relationship Ka × Kb = Kw, where Kw is the ion product of water. This means that strong acids (high Ka) will have weak conjugate bases (low Kb) and vice versa.
Examples & Analogies
Think of Ka and Kb like a seesaw. If one side (say, the acid side with Ka) is raised high, the other side (the base side with Kb) is lowered, demonstrating that if one side is strong, the other is weak due to the balancing nature of their relationship.
Example with Ammonium Ion and Ammonia
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
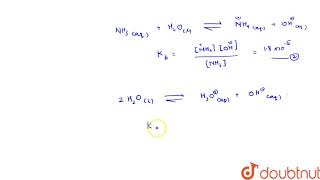
Considering the example of NH4+ and NH3 we see, NH4+(aq) + H2O(l) H3O+(aq) + NH3(aq) Ka = [H3O+][ NH3] / [NH4+] = 5.6 × 10–10 NH3(aq) + H2O(l) NH4+(aq) + OH–(aq) Kb =[ NH4+][ OH–] / NH3 = 1.8 × 10–5
Detailed Explanation
In this equilibrium involving the ammonium ion (NH4+) and ammonia (NH3), the dissociation of ammonium ion into ammonia and hydronium ions has a constant value Ka which is relatively low because NH4+ is not a very strong acid. Conversely, ammonia, when it acts as a base, has a Kb value that is higher than Ka, illustrating its properties as a base. When calculating the solubility product, Kw is equal to the product of these constants (Ka and Kb). Using this relationship, if we know Ka, we can calculate Kb using the formula Kb = Kw / Ka.
Examples & Analogies
Imagine you have a team of two players: a strong forward (the acid NH4+) and a clever defender (the base NH3). The better the forward plays (higher Ka), the less effective the defender is in scoring against (lower Kb). This showcases their interdependence based on their strengths.
Generalization of Ka and Kb Relationship
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It can be seen from the net reaction that the equilibrium constant is equal to the product of equilibrium constants Ka and Kb for the reactions added. Thus, Ka × Kb = Kw.
Detailed Explanation
The equation Ka × Kb = Kw encapsulates the relationship between acidity and basicity for conjugate pairs. By realizing that Kw is a constant at a given temperature (which equals 1.0 × 10^-14 at 25°C), we can understand that knowing either Ka or Kb allows us to easily determine the other.
Examples & Analogies
Consider Ka and Kb like two friends sharing a budget (Kw). If one friend spends more (high Ka), the other has to spend less to keep the total budget the same (low Kb). Thus, their financial decisions are interrelated, reflecting the relationship between acids and bases.
Key Concepts
-
Relationship between Ka and Kb: Ka × Kb = Kw.
-
Conjugate pairs: Strong acids have weak conjugate bases and vice versa.
-
Conversion of one constant to another allows for predicting behavior in acid-base reactions.
Examples & Applications
If a strong acid has a Ka of 1.0 × 10^6, its conjugate base would have a Kb less than 1.0 × 10^{-14}.
For the conjugate acid-base pair NH4+ and NH3, if Ka = 5.6 × 10^−10, Kb can be calculated as Kb = Kw / Ka.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
If Ka is high, acid is strong; weak conjugate base won't last long.
Stories
Imagine two friends, Ka and Kb, they dance together in a party that celebrates water but always keep Kw close by.
Memory Tools
K = Acid strength × Base strength = Kw (Ka × Kb = Kw).
Acronyms
Know Acids & Bases = Ka & Kb = Kw.
Flash Cards
Glossary
- Ka
The acid dissociation constant; quantifies the strength of an acid in solution.
- Kb
The base dissociation constant; quantifies the strength of a base in solution.
- Kw
The ion product of water; a constant at a given temperature (1.0 × 10^−14 at 25°C).
- Conjugate AcidBase Pair
A pair consisting of an acid and its corresponding base that differ by one proton.
Reference links
Supplementary resources to enhance your learning experience.
