Effect of a Catalyst
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Role of Catalysts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’ll begin by discussing catalysts. Can anyone explain what a catalyst is?

Isn’t it something that speeds up a chemical reaction?

Exactly! A catalyst accelerates a reaction without being consumed. It provides an alternative pathway that lowers the energy needed for the reaction to occur.

So, if it doesn’t get used up, why does it matter in terms of equilibrium?

Good question! While a catalyst speeds up both the forward and reverse reactions equally, it does not change the concentrations of reactants and products at equilibrium. Let’s remember this with the acronym 'CATS': Catalysts Accelerate both directions but don’t Shift the equilibrium. Can anyone recall an example of where we use catalysts?

In the Haber process for ammonia synthesis, right?

Precisely! Fritz Haber used iron as a catalyst to make ammonia production efficient.

And the conditions are optimized for the reaction?

Yes! That’s crucial for maximizing yield. Remember, catalysts make chemical processes faster and more efficient.
Effects on Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s dive deeper into the impact of catalysts on equilibrium. Can someone summarize what happens when we add a catalyst to a reaction at equilibrium?

It helps the reaction reach equilibrium faster but doesn’t change the equilibrium position.

Exactly! So, the equilibrium constant remains unchanged. Who can explain why 'catalysts do not affect equilibrium concentrations' in their own words?

Because a catalyst provides shortcuts for both forward and reverse reactions equally, right?

Well said! This means that if we have a reversible reaction, both paths are boosted. How might this concept be beneficial in industrial settings?

It allows industries to produce more products in less time which is more cost-efficient.

Correct! So remember, catalysts help in efficiency, not in altering outcomes.
Example of Catalyst in Industry
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To cement our understanding, let’s discuss an example. How does the iron catalyst work in the Haber process?

It increases the reaction rate between nitrogen and hydrogen to form ammonia.

Right! It specifically allows the reaction to occur at lower temperatures. Why is that important?

Lower temperatures save energy costs but might slow down reactions without a catalyst.

Excellent point! Now let’s consider this closing thought: Catalysts don’t solve every problem in chemistry. What might be a limitation?

If the equilibrium constant is very small, the catalyst wouldn’t help much, right?

Correct! In such cases, no matter how much we speed up the process, we might still end up with little to no product.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the role of catalysts in chemical reactions, emphasizing how they facilitate faster attainment of equilibrium by lowering activation energy for both forward and reverse reactions, while not altering the final concentrations of reactants and products.
Detailed
In chemical reactions, a catalyst serves as a substance that accelerates the rate of the reaction by providing an alternative pathway with lower activation energy. In this section, we highlight the mechanisms by which a catalyst works and clarify that it affects the rates of both the forward and reverse reactions equally, thereby not influencing the overall equilibrium position. Using the example of ammonia synthesis, we explain how Fritz Haber’s introduction of iron catalysts enabled industrial processes to operate efficiently at temperatures where overall yields would otherwise be unfavorable. The section emphasizes that while catalysts can enhance the speed of reactions to reach equilibrium, they do not affect the equilibrium constant or the proportions of reactants and products at equilibrium.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Catalysts
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
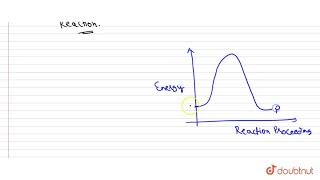
A catalyst increases the rate of the chemical reaction by making available a new low energy pathway for the conversion of reactants to products.
Detailed Explanation
Catalysts are substances that accelerate chemical reactions but are not consumed or changed during the reaction. They function by providing an alternative reaction pathway that has a lower activation energy than the uncatalyzed reaction. This means that more reactant molecules can overcome the energy barrier, leading to an increased rate of reaction.
Examples & Analogies
Imagine you are hiking a steep mountain. If there is a well-defined path that winds around the mountain, you can reach the top more easily than if you take a direct, steep route. The well-defined path is similar to what a catalyst does in a chemical reaction—it provides a more efficient route for the reactants to convert into products.
Catalyst Effects on Forward and Reverse Reactions
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It increases the rate of forward and reverse reactions that pass through the same transition state and does not affect equilibrium.
Detailed Explanation
By lowering the activation energy, a catalyst speeds up both the forward and reverse reactions equally. This means that while the reaction rate increases, the position of the equilibrium remains unchanged. The catalyst does not alter the equilibrium composition—it only allows the system to reach equilibrium faster.
Examples & Analogies
Think of a racing circuit where both cars are racing in opposite directions. If you set up a shortcut for both cars, they both would reach their destination quicker, but the results of the race (who wins) remain unchanged. In the same way, a catalyst allows the reactions to occur faster without changing the final state of the reaction mixtures.
Catalysts and Reaction Conditions
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Catalyst lowers the activation energy for the forward and reverse reactions by exactly the same amount.
Detailed Explanation
Regardless of the direction of the reaction, a catalyst facilitates both paths similarly by providing a stable intermediate (transition state) that lowers the energy barrier. This property ensures that neither direction is favored, preserving the ratio of reactants to products at equilibrium, even if the rate of reaching that state changes.
Examples & Analogies
Consider a bridge that connects two sides of a river. Whether you are going to the left or to the right, using the bridge makes the crossing much easier and faster, without changing how many people are going in either direction. That's what a catalyst does—facilitates the journey without changing the destination.
Case Study: Haber Process and Optimal Conditions
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
German chemist, Fritz Haber discovered that a catalyst consisting of iron catalyzes the reaction to occur at a satisfactory rate at temperatures, where the equilibrium concentration of NH3 is reasonably favourable.
Detailed Explanation
The Haber Process for ammonia synthesis is a practical application of catalysts in industry. The reaction between nitrogen and hydrogen to form ammonia is exothermic and slow without a catalyst. By introducing iron as a catalyst, the reaction rate increases significantly, allowing for higher production of ammonia under economically feasible conditions—around 500°C and 200 atm.
Examples & Analogies
Consider a factory assembly line. If you have machines (catalysts) that speed up each step, the entire process of assembling products speeds up, allowing you to produce more items in the same amount of time without changing the design of the items. This analogy reflects how catalysts enhance production efficiency in chemical manufacturing.
Limitations of Catalysts
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Catalyst does not affect the equilibrium composition of a reaction mixture.
Detailed Explanation
While catalysts are effective at speeding up chemical reactions, they do not change the final concentrations of reactants and products at equilibrium. This is an important limitation, as it means that while catalysts can increase the speed of reaching equilibrium, they cannot create more products than would be available without them.
Examples & Analogies
Imagine setting a timer for a certain amount of time when baking a cake. The timer helps you manage your time better, allowing the cake to be done faster, but it won’t change the recipe or how much cake you ultimately have. Similarly, catalysts enhance the speed but do not change the yield of a reaction.
Key Concepts
-
Catalysts accelerate the rates of both reactions.
-
Catalysts do not affect the position of equilibrium.
-
Catalytic processes are essential in industry.
Examples & Applications
The Haber process for ammonia production utilizes an iron catalyst.
Catalysts can be used to speed up reactions in petroleum refining.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
A catalyst in the mix, speeds up the fix, it aids the dance, without a chance to advance.
Acronyms
CATS - Catalysts Always speed up but don't Shift equilibrium.
Stories
In a bustling factory, a wise old cat named Catalyst ensured every reaction was swift. It guided the workers (the molecules) through shortcuts, allowing them to create products faster without changing who's who in their chemistry.
Memory Tools
Remember: Catalysts Control thermal efficiency without changing Equilibriums with their aid.
Flash Cards
Glossary
- Catalyst
A substance that increases the rate of a chemical reaction without being consumed by providing an alternative pathway.
- Equilibrium
The state in which the concentrations of reactants and products remain constant over time in a reversible reaction.
- Activation energy
The minimum energy that must be input to a chemical system for a reaction to occur.
- Haber process
An industrial method for synthesizing ammonia from nitrogen and hydrogen using a catalyst.
- Iron catalyst
A metal used in the Haber process to increase the rate of ammonia production.
- Equilibrium constant
A number that quantifies the ratio of product concentrations to reactant concentrations at equilibrium.
Reference links
Supplementary resources to enhance your learning experience.
