Calculating Equilibrium Concentrations
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Equilibrium Calculations
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to learn how to calculate equilibrium concentrations in chemical reactions. Can anyone tell me what equilibrium means in a chemical context?

Isn't it when the rate of the forward reaction equals the rate of the reverse reaction?

Exactly! At equilibrium, the concentrations of reactants and products remain constant. Let's break down how to calculate these concentrations.

What is the first step in this calculation?

The first step is to write the balanced chemical equation. This is crucial because it shows the stoichiometric ratios we need.
Creating an ICE Table
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we have our balanced equation, we can set up an ICE table. Who can remind us what ICE stands for?

Initial, Change, and Equilibrium concentrations!

That's right! We start by filling out the initial concentrations of our reactants and products. Let's say we have 1 M of A and 2 M of B in the reaction A + 2B ⇌ C. How would we start our ICE table?

We would write 1 for A and 2 for B in the first row!

Exactly! Next, we'll define the changes that occur as the reaction reaches equilibrium. We can denote this change with 'x'. For our example, what would the changes look like?

For A, it would be -x; for B, it would be -2x; and for C, it would be +x.

Correct! This forms the basis for our equilibrium row.
Using Equilibrium Expressions to Solve for x
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Once we have our ICE table established, we can plug in our equilibrium values into the equilibrium expression. Can anyone tell me what the expression looks like?

It's Kc = [C] / [A][B]^2 for the example we discussed!

Exactly! As we substitute the equilibrium concentrations from our ICE table into the Kc expression, we can solve for 'x'. What if the equation turns out to be quadratic?

Then we need to pick the solution that makes sense for our context, right?

That's right! Always choose the solution that fits physically. Finally, we calculate the equilibrium concentrations to find what they are at equilibrium.
Example Calculation
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's do an example together! For the reaction A ⇌ 2B, if the initial concentrations of A is 0.1 M and at equilibrium, the concentration of B is found to be 0.4 M, how can we find Kc?

I think we can set up our ICE table with A going to zero and then calculate x based on the changes!

Exactly! And then we substitute back to find Kc. Also, remember, verification is essential for our results. How can we verify our solution?

By checking if our Kc value is consistent with what we calculated from the concentrations!

Great job, everyone! This step will help us ensure our calculations are accurate.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides a structured approach to determining equilibrium concentrations using the balance of initial concentrations and stoichiometry. It emphasizes a step-by-step method, including establishing equilibrium expressions and applying them to solve for unknowns.
Detailed
Detailed Summary
In this section, we focus on calculating equilibrium concentrations in chemical reactions. When a reversible reaction reaches equilibrium, the concentrations of reactants and products remain constant, though individual molecules continue to react. To calculate these concentrations, the following steps are employed:
- Write the Balanced Equation: Identify the chemical equation governing the reaction. This forms the basis for determining the stoichiometric relationships between reactants and products.
- Set Up an ICE Table: Under the balanced equation, create an ICE (Initial, Change, Equilibrium) table for each substance involved in the reaction, where:
- Initial concentrations are recorded.
- Changes in concentration toward equilibrium are defined using a variable, commonly denoted as x or S (the solubility).
- Equilibrium concentrations are calculated from these relationships.
- Substitute into the Equilibrium Expression: Insert the equilibrium concentrations into the equilibrium expression (Kc).
- The expression is defined as Kc = [Products]/[Reactants], with concentrations raised to their stoichiometric coefficients.
- Solve for x: If the resulting equation is quadratic, choose the physically meaningful solution, ensuring it aligns with the context of the problem.
- Determining Final Concentrations: Calculate the equilibrium concentrations by substituting x back into the expressions.
- Verification: Optional, yet recommended — check the results by substituting the equilibrium values back into the equilibrium expression to confirm consistency with Kc.
This systematic approach is crucial for predicting the outcome of reactions, determining yield, and understanding chemical behavior in various contexts.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Step 1: Write the Balanced Equation
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In this step, one must write the balanced equation for the reaction being studied.
Detailed Explanation
Writing a balanced chemical equation is crucial as it provides the stoichiometry, indicating how many moles of each reactant and product are involved. For example, in a reaction where A and B produce C and D, the balanced equation might look like this: A + B ⇌ C + D. This forms the foundation for subsequent calculations of concentrations.
Examples & Analogies
Consider a recipe for a cake; just as you need the right proportions of ingredients to achieve the desired flavor and texture, you must know the correct stoichiometric ratios in a chemical equation to predict how much product will be formed.
Step 2: Construct a Concentration Table
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Construct a table that includes the initial concentrations, the changes in concentration as the reaction approaches equilibrium, and finally, the equilibrium concentrations.
Detailed Explanation
This table allows you to visualize how the concentrations of the substances change as the reaction progresses. You define 'x', a variable representing the change in concentration of a particular reactant or product. Using the stoichiometric coefficients from the balanced equation, you can express the concentrations of all other species in terms of x. For example:
| Species | Initial Concentration | Change | Equilibrium Concentration |
|---|---|---|---|
| A | [A]0 | -x | [A]0 - x |
| B | [B]0 | -y * x | [B]0 - y * x |
| C | 0 | +x | x |
| D | 0 | +y * x | y * x |
Examples & Analogies
Think of tracking the number of people entering and leaving a party. You can set up a chart that records the initial number of guests (initial concentration), how many leave (change), and how many guests are present at the end (equilibrium concentration).
Step 3: Substitute and Solve for x
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Substitute the equilibrium concentrations into the equilibrium expression and solve for x.
Detailed Explanation
Once you have expressed all concentrations in terms of x, you can substitute these values into the equilibrium constant expression. For example, if Kc = [C][D] / ([A][B]), and you have defined all concentrations in terms of x, you can solve for x. This often involves working with a quadratic equation if the stoichiometry is not 1:1.
Examples & Analogies
It's like solving for the unknowns in a budget after tracking expenditures. By using the equations from your budget (equilibrium expression) and plugging in numbers (substituting concentrations), you can find out how much money you have left after spending.
Step 4: Calculate Equilibrium Concentrations
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Using the value of x obtained from the previous step, calculate the equilibrium concentrations of all species.
Detailed Explanation
With the value of x determined, you can easily find the equilibrium concentrations by plugging x back into your expressions for each species. If you defined that A changes by -x and C changes by +x, simply substituting x gives you the final equilibrium concentrations.
Examples & Analogies
This stage is similar to figuring out how much cake you will have left after slices are removed. After calculating how much has been taken (x), you just subtract from your total to find what you have remaining.
Step 5: Check Your Results
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Finally, verify your calculations by substituting the calculated equilibrium concentrations back into the equilibrium equation.
Detailed Explanation
This step ensures your work is reliable. By plugging your calculated values of concentrations back into the equilibrium expression, you should find that both sides of the equation balance, confirming your calculations were correct.
Examples & Analogies
It's like double-checking your math after solving a problem; you want to ensure that your answer is consistent and correct before moving on.
Key Concepts
-
Equilibrium Concentration: The concentration of reactants and products remains constant in a dynamic balance.
-
ICE Table: A systematic way to track the initial, change, and equilibrium concentrations of reactants and products.
-
Kc: The equilibrium constant that gives the ratio of concentrations of products to reactants.
Examples & Applications
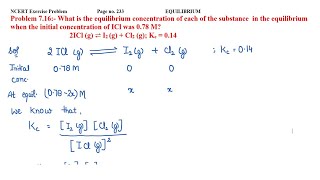
Example 1: Calculate the equilibrium concentration of NO2 if [NO] = 0.3 M and [O2] = 0.1 M for the reaction 2NO ⇌ O2 + 2NO2.
Example 2: Given the initial concentrations and equilibrium concentration of products, determine Kc for the reaction A + B ⇌ C + D.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When at equilibrium, all must be still, Rates equal out, it's a chemical thrill!
Stories
Imagine a seesaw; if one side is heavier, the other has to give way until balance is restored, just like in reactions!
Memory Tools
ICE: Initial, Change, Equilibrium helps you remember how to set up your table.
Acronyms
Kc Stands for Koncentration Chaos at equilibrium!
Flash Cards
Glossary
- Equilibrium
A state in a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction.
- ICE Table
A table used to calculate the concentrations of reactants and products at equilibrium, showing their Initial, Change, and Equilibrium concentrations.
- Equilibrium Expression
An expression that relates the concentrations of reactants and products at equilibrium, generally in the form Kc.
- Kc
The equilibrium constant for a reaction expressed in terms of concentration.
- x
A variable representing the change in concentration of reactants and products as a reaction reaches equilibrium.
Reference links
Supplementary resources to enhance your learning experience.
