Predicting the Extent of a Reaction
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Reaction Extent
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today we will discuss predicting the extent of a reaction using equilibrium constants. So, what do you think happens when the number of reactants equals the number of products at equilibrium?

That means the reaction has stopped?

Not quite! At equilibrium, the rates of the forward and reverse reactions are equal. This means that even though concentrations don't change, both reactions are still occurring.

So does that mean we can predict how far a reaction will go?

Exactly! We can use equilibrium constants to predict the extent of a reaction. For example, if Kc is greater than 1000, products strongly dominate the equilibrium mixture, indicating that the reaction proceeds nearly to completion.
Understanding Equilibrium Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Kc and Kp are crucial for understanding chemical equilibria. What do you think the difference is between the two?

Is Kc for concentrations and Kp for pressures?

That's correct! Kc uses molar concentrations, while Kp uses the partial pressures of gaseous components at equilibrium.

How do we use these values in practice?

Good question! By calculating Kc or Kp, we can determine the direction a reaction will shift when conditions change.
Evaluating Reaction Direction
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s explore what happens when we change concentrations or pressure. If we increase the concentration of a reactant, what does Le Chatelier's principle suggest will happen?

The reaction will shift to the right to form more products?

Exactly! Any increase in reactant concentration pushes the equilibrium position forward, favoring product formation. And if we increase pressure in a gaseous reaction, the reaction favors the side with fewer gas moles.

What if we decrease concentration?

That's correct! Decreasing the concentration of a product will shift the equilibrium to the product side to replenish what was lost. This concept is vital in industrial processes.
Applications of Equilibrium Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we've covered the basics, how do you think this applies to industries, like in chemical manufacturing?

Industries can adjust concentrations to get more product, right?

Absolutely! By manipulating conditions such as temperature and pressure, industries can optimize production for reactions with high Kc values.

Are there situations where we wouldn't want the reaction to go to completion?

Yes! Some reactions may be reversible and it's sometimes advantageous to maintain a balance of reactants and products.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains the significance of equilibrium constants in determining the extent to which a reaction proceeds, illustrating that high values of Kc or Kp indicate predominant product formation, while low values suggest minimal product formation.
Detailed
Understanding the Extent of a Reaction
Chemical equilibrium is fundamental in both biological and environmental processes. This section focuses on understanding how to predict the extent of a reaction through the use of equilibrium constants, known as Kc and Kp.
When a reaction reaches equilibrium, the concentrations of reactants and products no longer change even though both forward and reverse reactions continue to occur. The relationship between these concentrations in a chemical reaction can be expressed by the equilibrium constant. The section lists three groups based on the extent of reaction:
- Group 1: Reactions that go nearly to completion with negligible concentrations of reactants remaining.
- Group 2: Reactions that produce only small amounts of products compared to the reactants.
- Group 3: Reactions in which reactants and products exist in comparable amounts.
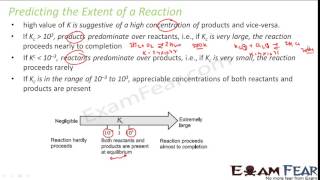
The equilibrium constant indicates the concentration ratios of products to reactants at equilibrium. If Kc is greater than 10^3, it signifies product predominance, suggesting that the reaction approaches completion. Conversely, if Kc is less than 10^-3, reactants dominate. Variations in conditions such as concentration and temperature can shift equilibrium positions, thereby affecting the extent of reactions.
This understanding is crucial in various applications, particularly in optimizing industrial processes and in laboratory settings to favor the formation of desired products.
Youtube Videos




Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Equilibrium and Reaction Extent
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The numerical value of the equilibrium constant for a reaction indicates the extent of the reaction. But it is important to note that an equilibrium constant does not give any information about the rate at which the equilibrium is reached.
Detailed Explanation
The equilibrium constant, represented as K_c or K_p, provides insights into how far a reaction proceeds before reaching equilibrium. A higher value of K suggests that the reaction favors the formation of products (the products are present in higher concentrations compared to the reactants). Conversely, a lower K value indicates that reactants are favored. However, K does not inform us about how quickly equilibrium is achieved; this depends on factors like temperature and the rate of the reactions involved.
Examples & Analogies
Think of K as a scoreboard in a sports game, showing which team is winning (products or reactants) but not how quickly a team scores (the rate of the reaction). Just like a high score indicates a lead, a high K indicates a favorable position for products.
Generalizations About Reaction Outcomes Based on K_c Values
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
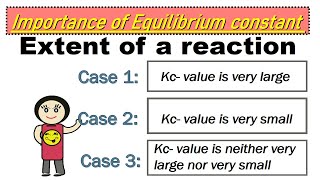
We can make the following generalizations concerning the composition of equilibrium mixtures: If Kc > 10^3, products predominate over reactants... If Kc < 10^–3, reactants predominate over products.
Detailed Explanation
These generalizations help predict the outcome of a chemical reaction. If K_c is greater than 1000 (10^3), it means that at equilibrium, there are far more products compared to reactants, indicating that the reaction favors product formation. On the other hand, if K_c is less than 0.001 (10^−3), it suggests that most of the substances present are the original reactants and that the reaction does not strongly favor the formation of products. If K_c is between 0.001 and 1000, both reactants and products are present in appreciable amounts.
Examples & Analogies
Imagine a pizza restaurant where K_c can be visualized as the popularity of pizza versus burgers on the menu. If K_c > 1000, it’s like everyone orders pizza (products), while if K_c < 0.001, burgers (reactants) remain on the table untouched. If K_c is between these values, diners are sharing both options equally.
CASE EXAMPLES FOR K_c VALUES
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consider the following examples...
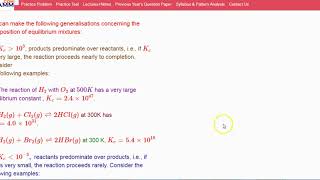
(a) The reaction of H2 with O2 at 500 K has a very large equilibrium constant, Kc = 2.4 × 10^47.
(b) H2(g) + Cl2(g) 2HCl(g) at 300K has Kc = 4.0 × 10^31.
(c) H2(g) + Br2(g) 2HBr (g) at 300 K, Kc = 5.4 × 10^18.
Detailed Explanation
In these examples, the extraordinarily high K_c values illustrate reactions that heavily favor the formation of products. For instance, in the first reaction of H2 and O2, the extremely high K suggests that nearly all substances at equilibrium consist of water (the product), indicating that the reaction goes nearly to completion.
Examples & Analogies
Picture a race to finish a marathon: a K value similar to 2.4 × 10^47 indicates that nearly everyone crossed the finish line before even a few participants dropped out! In the context of our reactions, we see that the 'winners' (products) dominate the track.
Low K_c Values Indicate Very Rare Reaction
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
If Kc < 10^–3, reactants predominate over products... These generalizations are illustrated in Fig. 6.6.
Detailed Explanation
When K_c values are low, it indicates that the reverse reaction is favored, suggesting that the reactants remain largely unreacted at equilibrium. This can occur in reactions that are not favorable under the given conditions, indicating the layout of products is minimal compared to reactants.
Examples & Analogies
If we think of a concert with limited seating (reactants) and only a few fans show up (products), a low K would mean that most still opted to stay home! The concert remains largely unfilled and the music producer knows this concert is hardly going to be a hit.
Key Concepts
-
Equilibrium: The state where the concentrations of reactants and products remain constant over time.
-
Kc and Kp: The constants representing the equilibrium concentrations or pressures of products and reactants.
-
Le Chatelier's Principle: A framework for predicting the effect of changes in conditions on the position of equilibrium.
Examples & Applications
If Kc is greater than 10^3, it suggests that products are favored in the reaction.
If Kc is less than 10^-3, the reactants predominate, indicating minimal reaction progress.
When temperature is increased in an exothermic reaction, the equilibrium will favor the endothermic direction.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In reactions, what’s the key? Kc shows products joyfully!
Stories
Imagine a dance where reactants twirl, adding more leads to a product swirl!
Memory Tools
Kc = C/P (Concentration over Product) to remember how equilibriums adjust!
Acronyms
LEAP - Le Chatelier, Equilibrium Adjusts Position.
Flash Cards
Glossary
- Equilibrium
A state in a reversible reaction where the rates of the forward and reverse reactions are equal.
- Equilibrium constant (Kc)
A constant value that expresses the relationship between the concentrations of products and reactants at equilibrium.
- Equilibrium constant (Kp)
A constant that expresses the relationship between the partial pressures of gaseous reactants and products at equilibrium.
- Le Chatelier's Principle
A principle that predicts how an equilibrium will shift in response to changes in concentration, pressure, or temperature.
Reference links
Supplementary resources to enhance your learning experience.
