Ionization Constants of Weak Acids
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Ionization Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll explore the ionization constants of weak acids, denoted as *Ka*. Can anyone tell me what we mean by 'ionization' in this context?

Is it about how much of the acid breaks down into ions when dissolved in water?

Exactly! The ionization constant, *Ka*, measures how well an acid dissociates in water to form ions. The greater the value of *Ka*, the stronger the acid.

So, if we have two acids, one with a larger *Ka* than the other, we can say the one with the larger *Ka* is the stronger acid?

Correct! Now, let’s remember that the ionization of a weak acid can be represented by the equation. What is the general formula for *Ka*?

It’s the concentration of the hydrogen ions times the concentration of the conjugate base over the concentration of the undissociated acid.

Perfect! It looks like you’re grasping the concept. Let’s proceed to how we derive these constants from experimental data.
Calculating Ionization Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

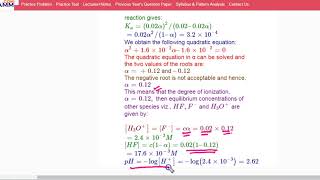
Let’s consider an example involving acetic acid. If we start with 0.02 M acetic acid and find its pH to be 2.88, how do we calculate *Ka*?

First, we need to calculate the concentration of hydrogen ions from the pH.

Exactly! Using the pH equation, how would we find [H+]?

We use [H+] = 10^(-pH). So for this pH 2.88, [H+] would be about 1.32 × 10^(-3) M.

That’s right! Now substituting values into the *Ka* expression will show us how we derive the ionization constant. What do we get?

Using *Ka = [H+][A-] / [HA]*, we plug in our values to find *Ka*.

Well done! Calculating the concentration and using it in our equation emphasizes the importance of knowing the equilibrium concentrations when dealing with weak acids!
Relationship Between Ka and Kb
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's talk about the relationship between *Ka*, the ionization constant of acids, and *Kb*, the ionization constant for bases. What do you think happens to these constants in a conjugate acid-base pair?

They must be connected somehow, right? Like if you know one, you can figure out the other?

You got it! The equation is *Ka × Kb = Kw*, where *Kw* is the ion product constant of water. This means knowing just one of these constants allows us to calculate the other.

So for example, if I had the *Ka* for acetic acid, could I find the *Kb* for acetate ion?

Exactly! Let’s do an example calculation to solidify this concept. If the *Ka* for acetic acid is 1.74 × 10^(-5), what would be the *Kb* for acetate?

Using the formula, I would calculate *Kb* as Kw / *Ka* so that would be *Kb = 10^(-14) / (1.74 × 10^(-5))*.

Right! This relationship allows us to predict how acids and bases behave in solution. Excellent work today!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section provides a comprehensive overview of the concept of ionization constants for weak acids, including how they influence the extent of ionization, the pH of solutions, and the relationships between the ionization constants of acids and their conjugate bases. Key equations to understand the behavior of weak acids in solution are also presented.
Detailed
Ionization Constants of Weak Acids
In the context of acid-base chemistry, the strength of a weak acid is represented by its ionization constant, denoted as Ka. This constant quantifies the equilibrium between a weak acid, its conjugate base, and hydronium ions in an aqueous solution, as given by the ionization reaction:
$$
HA(aq) + H_2O(l) \rightleftharpoons H_3O+(aq) + A^-(aq)
$$
The expression for the ionization constant is derived from the equilibrium concentrations of the reactants and products:
$$
K_a = \frac{[H_3O^+][A^-]}{[HA]}
$$
The value of Ka indicates the degree of dissociation of the acid; a larger Ka value signifies a stronger acid as it indicates greater ionization in solution. The section also discusses the methodology to calculate the ionization constants and pH levels for weak acids through algebraic manipulation of the ionization equations.
Furthermore, it covers the concept of the conjugate base's ionization constant (Kb) and the relationship between Ka and Kb for conjugate acid-base pairs:
$$
K_a \times K_b = K_w
$$
where Kw is the ion product constant of water. This relationship enables calculations of acid strengths from known base strengths and vice versa. Overall, understanding ionization constants is fundamental for evaluating the behavior of weak acids in biological and chemical systems.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Weak Acid Ionization Equilibrium
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consider a weak acid HX that is partially ionized in the aqueous solution. The equilibrium can be expressed by:
HX(aq) + H2O(l) H3O+(aq) + X–(aq)
Initial concentration (M):
- c 0 0
Let α be the extent of ionization
Change (M):
- cα +c α +c α
Equilibrium concentration (M):
- c-cα c α c α
Detailed Explanation
In this section, we discuss how weak acids like HX dissociate to form ions in solution. The acid dissociation occurs at equilibrium, meaning the amount of dissociated ions balances out with the undissociated acid over time. The extent of dissociation (denoted as α) represents the fraction of the acid molecule that ionizes. Here, 'c' is the initial concentration of the undissociated acid. At equilibrium, we have less of the original acid (c - cα) and equal amounts of the ions produced (cα). A weak acid does not fully ionize, so a significant proportion remains as undissociated molecules.
Examples & Analogies
Think of a weak acid like acetic acid (vinegar) dissolving in water. When you first mix it, not all molecules split into ions. If you could visualize the solution, you would see a lot of intact acetic acid molecules alongside some ions (H3O+ and acetate ions). Unlike a strong acid like hydrochloric acid, which splits completely, acetic acid maintains some of its molecules intact because it's a weak acid.
Dissociation Constant (Ka)
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Using these notations, we can derive the equilibrium constant for the above discussed acid-dissociation equilibrium:
Ka = c2α2 / c(1-α) = cα2 / 1-α
Ka is called the dissociation or ionization constant of acid HX. It can be represented alternatively in terms of molar concentration as follows,
Ka = [H+][X–] / [HX]
Detailed Explanation
The ionization constant (Ka) is a crucial measure of how strong a weak acid is. It quantifies the extent to which an acid ionizes in solution. The formula shows that Ka is derived from the concentration of the ions produced ([H+] and [X–]), and the concentration of the remaining undissociated acid ([HX]). A larger Ka indicates a stronger acid because it means more of the acid has ionized into its constituent ions. Conversely, a smaller Ka means less ionization and thus a weaker acid.
Examples & Analogies
Imagine a classroom filled with students, some of them are actively participating in discussions (these represent the ions), while others are quietly observing the class (these represent the undissociated acid). If more students start participating (higher Ka), it implies that the class is engaging more deeply, just as a higher Ka indicates a stronger acid that ionizes more in solution.
Strength of Weak Acids
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At a given temperature T, Ka is a measure of the strength of the acid HX; i.e., larger the value of Ka, the stronger is the acid. Ka is a dimensionless quantity with the understanding that the standard state concentration of all species is 1M.
Detailed Explanation
In this portion, the relationship between the ionization constant and acid strength is made clear. As the value of Ka increases, it reflects a higher tendency for the acid to donate protons to the solution, thereby indicating that the acid is stronger. The statement about being a 'dimensionless quantity' means that Ka is useful for comparing the strengths of different acids under standardized conditions (1M concentration).
Examples & Analogies
Think of Ka like the score in a competition; the higher the score (Ka), the better the performance (strength of the acid). If one acid has a Ka of 1.0 and another has 0.1, the first acid is 'scoring' much higher, indicating it is stronger and more willing to give up its protons.
Values of Ionization Constants
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The values of the ionization constants of some selected weak acids are given in Table 6.6.
Detailed Explanation
This is a straightforward reference point where the specific values of Ka for various weak acids are provided. These values serve as benchmarks to understand how different acids compare in terms of their strength and how they behave in aqueous solutions. The table categorizes acids by their ionization constants so one can easily discern which acids are stronger or weaker.
Examples & Analogies
If you think of a leaderboard in a sports tournament, the table of ionization constants works the same way, showcasing the strengths of different acids much like comparing athletes by their scores. Just as the leaderboard allows fans to see which teams or players are performing well, the table of K values allows chemists to quickly assess the relative strength of various acids.
Key Concepts
-
Ionization of weak acids produces hydronium ions and conjugate bases.
-
Ka is a measure of acid strength, where larger Ka indicates a stronger acid.
-
Relationship between Ka and Kb allows for the calculation of ionization constants for conjugate acids and bases.
-
pH is calculated using the concentration of hydronium ions, which directly relates to Ka of the weak acid.
Examples & Applications
If acetic acid has an initial concentration of 0.1M, and the pH of the solution is measured to be 4.75, we can calculate Ka using the formula Ka = [H+][Ac-]/[HA].
When hydrochloric acid (a strong acid) is fully dissociated in water, it produces H3O+ ions completely, while a weak acid like acetic acid only partially dissociates.
Memory Aids
Interactive tools to help you remember key concepts
Memory Tools
Remember the phrase 'King Acid' for Ka relates to how strong an acid is.
Stories
Imagine an acid trying to ionize in water, with a strong acid waving goodbye as it fully dissociates while a weak acid hesitantly releases some ions.
Acronyms
Use 'KAs' to remember 'KA = acid strength'.
Rhymes
For acids weak and strong, Ka will help you along.
Flash Cards
Glossary
- Ionization Constant (Ka)
The equilibrium constant for the dissociation of a weak acid in water, indicating its strength.
- Conjugate Base
The species that remains after an acid donates a proton.
- Equilibrium Concentration
The concentrations of reactants and products in a chemical reaction that does not change over time.
- Hydronium Ion (H3O+)
The ion formed when a proton is added to a water molecule; it is commonly used to represent acidity in a solution.
- Dissociation
The process by which a compound breaks down into its constituent ions in solution.
Reference links
Supplementary resources to enhance your learning experience.
