HETEROGENEOUS EQUILIBRIA
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Heterogeneous Equilibria
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Welcome, class! Today we'll discuss heterogeneous equilibria. Can anyone tell me what this means?

Is it when different phases of matter, like solids and gases, are involved in a reaction?

Exactly! Heterogeneous equilibria occur in systems with multiple phases. A good example is water vapor and liquid water. Can anyone provide the chemical equation for this?

H2O(l) ⇌ H2O(g)!

Great job! Now, why do we not consider the concentration of liquids or solids in equilibrium expressions?

Because their concentration remains constant?

Exactly, and that leads us to equilibrium constants. Let's summarize this point: pure solids and liquids do not appear in Kc expressions.
Equilibrium Constants for Heterogeneous Systems
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss how to derive equilibrium constants for these equilibria. For instance, consider the reaction for calcium hydroxide...

"Ca(OH)2(s) ⇌ Ca2+(aq) + 2OH−(aq).
Common Ion Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now let's look at the common ion effect. Who can explain what it is?

Isn't it when the addition of an ion shifts the equilibrium position, affecting solubility?

That's correct! For instance, when we add sodium acetate to a solution of acetic acid, what happens to the equilibrium?

It shifts towards the undissociated acetic acid!

Well done! This principle is critical in applications like buffer solutions. Let’s summarize the key points.
Solubility Product Constant
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s now examine the solubility product constant, Ksp, particularly for sparingly soluble salts, such as barium sulfate.

How do we express Ksp for these salts?

Good question! For BaSO4, the Ksp expression would be [Ba2+][SO42–]. What does this mean for its solubility?

It indicates how much of the salt will dissolve to achieve equilibrium.

Exactly! It’s an essential part of understanding environmental chemistry. Let’s put this knowledge to the test.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses heterogeneous equilibria involving different phases in chemical systems. It explains how equilibrium constants are derived for systems with solutes and solvents, emphasizing that the concentration of pure solids and liquids is constant and how it impacts equilibrium expressions. The concept of common ion effect and its practical importance for solubility product constants are also covered.
Detailed
Heterogeneous Equilibria
Heterogeneous equilibria involve systems where substances exist in multiple phases, such as solid, liquid, and gas. A classic example is the equilibrium established between water vapor and its liquid form, represented as:
H2O(l) ⇌ H2O(g)
In this case, the equilibrium constant can be expressed in terms of the concentrations of the gaseous and liquid phases.
Significance of the Equilibrium Constant
For reactions involving solids or liquids, their concentrations do not appear in the equilibrium expression as they remain unchanged; hence, we utilize only the concentrations of gaseous or aqueous species. For instance, considering the dissociation of calcium hydroxide:
Ca(OH)2(s) ⇌ Ca2+(aq) + 2OH−(aq)
The equilibrium constant expression would be:
Kc = [Ca2+][OH−]²
The constant nature of the concentration of solids or pure liquids is essential in analyzing the dynamic balance in heterogeneous systems.
Common Ion Effect and Solubility Product Constant
The common ion effect demonstrates how the solubility of a salt decreases upon the addition of a common ion. For example, adding sodium acetate to acetic acid increases acetate ion concentration, shifting equilibrium back toward the undissociated acid—this principle is applied in various chemical analyses and processes. The solubility product constant (Ksp) reflects the solubility levels of sparingly soluble salts in water and influences precipitation and dissolution dynamics.
Understanding these principles is key in fields such as environmental chemistry and material science, where reactions in different phases are common.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Heterogeneous Equilibrium
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
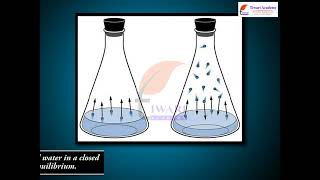
Equilibrium in a system having more than one phase is called heterogeneous equilibrium. The equilibrium between water vapour and liquid water in a closed container is an example of heterogeneous equilibrium.
H2O(l) H2O(g)
Detailed Explanation
Heterogeneous equilibrium occurs in systems where multiple phases (solid, liquid, gas) are involved. In the given example, water in its liquid form and its vapor form exists in equilibrium in a closed container. This means that both states coexist, and there is an ongoing exchange between the two, where some water molecules evaporate into vapor and others condense back into liquid.
Examples & Analogies
Think of a sealed jar of water. You notice that on the inside of the jar, there are droplets on the glass. This is because some of the water has evaporated into vapor, but water droplets are condensing back on the glass. This bustling exchange between liquid water and water vapor inside the jar is a perfect illustration of heterogeneous equilibrium.
Example of Heterogeneous Equilibrium
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In the same way, equilibrium between a solid and its saturated solution, Ca(OH)2 (s) + (aq) Ca2+ (aq) + 2OH– (aq) is a heterogeneous equilibrium.
Detailed Explanation
Here, calcium hydroxide is a solid that partially dissolves in water to form a saturated solution where calcium ions and hydroxide ions exist in equilibrium with undissolved solid. This showcases that a solid can be in equilibrium with its ions in solution—a clear characteristic of heterogeneous equilibria.
Examples & Analogies
If you’ve ever made a saturated solution of salt in water, you would have seen salt at the bottom of the container while some dissolved in the liquid. The undissolved salt represents the solid phase, while the dissolved salt in the water represents the ionic form. The balance between the salt dissolving and the solid remaining at the bottom is a practical example of heterogeneous equilibrium.
Simplifying Equilibrium Expressions
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Heterogeneous equilibria often involve pure solids or liquids. We can simplify equilibrium expressions for the heterogeneous equilibria involving a pure liquid or a pure solid, as the molar concentration of a pure solid or liquid is constant (i.e., independent of the amount present).
Detailed Explanation
In equilibrium expressions, concentrations of pure solids or liquids can be excluded since they do not change regardless of the amount present. For example, if solid calcium hydroxide is involved in the reaction, its concentration is not included in the equilibrium expression because it remains constant. This makes equations simpler and focuses on the species that do affect the equilibrium.
Examples & Analogies
Imagine baking a cake. The amount of flour (a solid) you use doesn’t change the overall flour component in the batter once it’s mixed in. Thus, when looking at how ingredients interact (like sugar or baking soda), you only need to focus on those that affect the batter’s consistency, similar to how we exclude solids from equilibrium calculations.
Thermal Dissociation Example
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let us take thermal dissociation of calcium carbonate which is an interesting and important example of heterogeneous chemical equilibrium.
CaCO3 (s) CaO (s) + CO2 (g)
Detailed Explanation
This reaction signifies that solid calcium carbonate dissociates thermally into solid calcium oxide and gaseous carbon dioxide. The gaseous product is included in the equilibrium expression, while the solids are not, demonstrating the principles of heterogeneous equilibrium.
Examples & Analogies
In a chemistry lab, upon heating calcium carbonate, you'll see the solid breaking down while gas escapes. The gas molecules interact with the environment differently than the solids, similar to how ice changes to water and then to vapor when heated, showing the different phases in equilibrium.
Equilibrium Constant for Heterogeneous Equilibrium
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
On the basis of the stoichiometric equation, we can write,
Kc = [CO2(g)]
Detailed Explanation
In this case, the equilibrium constant can be simplified to reflect only the concentrations of the gaseous products since solids are excluded. Thus, the equilibrium constant Kc is expressed only in terms of the concentration of carbon dioxide in the gas phase, emphasizing the nature of heterogeneous equilibrium where only the active phases contribute to the equilibrium concentrations.
Examples & Analogies
If you think about opening a soda can, the gas (carbon dioxide) bubbles out, while the soda remains liquid. The amount of gas escaping determines the fizz, similar to how the gaseous concentration impacts the equilibrium but the liquid part (soda) remains constant in this analogy.
Key Concepts
-
Heterogeneous Equilibrium: Involves multiple phases and is dynamic.
-
Equilibrium Constant Kc: Ratio of product to reactant concentrations at equilibrium.
-
Common Ion Effect: Addition of an ion reduces solubility.
-
Ksp: Specific equilibrium constant for sparingly soluble salts.
Examples & Applications
The dissolution of BaSO4 leading to Ba2+ and SO42- ions demonstrates Ksp.
Adding sodium chloride to a solution of silver chloride decreases its solubility due to the common ion effect.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
A liquid’s in phase, if solid's also there, the K is a ratio; we keep that in care.
Stories
Imagine a party with ball pits (solids) all around. Gases keep mingling (equilibrium), ensuring no one feels out of bounds.
Memory Tools
Remember: 'SOLIDS Stay Out' when making K expressions - they don't change concentration.
Acronyms
KSP
for Kinetic
for Sparingly soluble
for Product constant.
Flash Cards
Glossary
- Heterogeneous Equilibrium
Equilibrium involving multiple phases, such as solid and liquid or gas.
- Equilibrium Constant (Kc)
A constant that expresses the ratio of concentrations of products to reactants at equilibrium.
- Common Ion Effect
The phenomenon where the addition of an ion affects the solubility of a sparingly soluble salt.
- Solubility Product Constant (Ksp)
The equilibrium constant for the solubility of a sparingly soluble salt.
Reference links
Supplementary resources to enhance your learning experience.
