Equilibrium in Physical Process
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we’ll explore the concept of equilibrium in physical processes. Can anyone tell me what they think equilibrium means?

Isn't it when things are balanced or stable?

Exactly! Equilibrium is achieved when opposing processes occur at the same rate. For example, when a liquid evaporates in a sealed container, the rate of molecules leaving the liquid equals those returning from the vapour phase.

So, it’s not like nothing happens, right? There’s still movement?

Correct! This dynamic nature means there's constant activity at the boundary of phases. Remember, equilibrium is all about balance!

How do changes like temperature affect this equilibrium?

Good question! An increase in temperature raises the rate of evaporation, resulting in a new equilibrium state. This connection is vital for understanding physical equilibria.
Phase Transformation Processes
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s discuss solid-liquid equilibria. What happens when ice and water are placed in a container?

They stay the same after a while, right? The amounts don’t change?

Exactly! At a specific temperature like 273K, ice and water can coexist. This is an example of dynamic equilibrium where both phases are in constant motion.

So, the ice melts, and some water might freeze back?

Precisely! It’s a continuous process. Now, can someone explain what happens when we look at liquid-vapour equilibria?

Isn't it when the liquid converts to vapor, and the vapor can also return to liquid?

Right! And at equilibrium, the vapour pressure remains constant at a specific temperature.
Factors Affecting Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Factors such as temperature and pressure play a crucial role in altering the equilibrium state. Can anyone share how increasing temperature might affect a liquid-vapour system?

It would increase the vapour pressure, right?

Exactly! And why is that?

Because more molecules would have enough energy to escape into the vapor phase!

Great insight! It's interesting to note how equilibrium not only applies to chemical reactions but also to physical processes.
Overall Summary of Equilibrium Concepts
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s recap what we’ve discussed about equilibrium in physical processes. What are the key takeaways?

Equilibrium is dynamic, involving constant movement at a microscopic level.

Temperature and pressure changes can shift the state of equilibrium!

Exactly! Remember, whether it’s melting ice, evaporating water, or the sublimation of solids, understanding the principles of equilibrium is essential in chemistry!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains how equilibrium is achieved in various physical processes like solid-liquid and liquid-vapour transitions. It emphasizes the dynamic aspect of these equilibria and introduces the concept of vapor pressure in relation to temperature and states of matter, concluding with principles driving physical equilibrium.
Detailed
Detailed Summary
This section delves into the dynamic nature of equilibrium in physical processes, exemplified through critical transitions such as solid-liquid, liquid-vapour, and solid-vapour states. The concept of equilibrium is central, defined as the state where the rate of processes in both directions becomes equal, leading to no net change in measurable properties over time.
Key Concepts
- Dynamic Equilibrium: This state is characterized by continuous processes where opposing reactions occur at the same rate. For instance, in a liquid-vapour system, the number of molecules evaporating equals those condensing.
- Phase Transformation: The section outlines phase changes that result in equilibrium, uncomplicated by temperature or pressure variations. Notably, for water, solid (ice) and liquid coexist at 273K, showcasing equilibrium dynamics.
- Liquid-Vapour Equilibrium: Discussing vapour pressure, the section illustrates how an increase in temperature raises root pressure, leading to more active evaporation until equilibrium is reached.
- Solid-Vapour and Solid-Liquid Equilibrium: Examples like sublimation, illustrated by iodine and camphor, demonstrate how solids can transition to gaseous states, emphasizing the balance needed for equilibrium.
- Dissolution Dynamics: Last but not least, heterogeneous equilibria involving dissolution are covered, cementing the necessity of understanding ionic interactions in solubility and reactions in balanced mixtures.
This comprehensive overview is critical not only for theoretical understanding but also for practical applications in chemistry, highlighting the intrinsic relationships that govern physical equilibria.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Equilibrium in Physical Processes
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
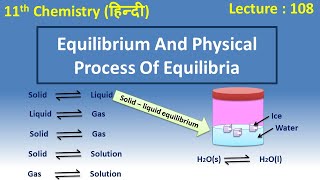
The characteristics of system at equilibrium are better understood if we examine some physical processes. The most familiar examples are phase transformation processes, e.g., solid liquid, liquid gas, solid gas.
Detailed Explanation
In physical processes, equilibrium is the state where the forward and reverse processes occur at equal rates. Key examples include changes in state, like the transition between solid and liquid or liquid and gas. At equilibrium, the amounts of each state do not change, even though molecular transitions are ongoing.
Examples & Analogies
Consider melting ice in water. Although the amount of ice and water remains constant at 0°C, molecules continuously move from ice to water and vice versa. This is like a see-saw; when one side goes down, the other goes up, but overall balance is maintained.
Solid-Liquid Equilibrium
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ice and water kept in a perfectly insulated thermos flask (no exchange of heat between its contents and the surroundings) at 273K and the atmospheric pressure are in equilibrium state and the system shows interesting characteristic features. We observe that the mass of ice and water do not change with time and the temperature remains constant. However, the equilibrium is not static. The intense activity can be noticed at the boundary between ice and water.
Detailed Explanation
At 273K, ice and liquid water coexist in equilibrium. Although the total mass of ice and water stays constant, individual molecules are continuously transitioning between the solid and liquid phases. Water molecules collide with ice, some freeze, while some ice melts, ensuring that the amounts remain unchanged.
Examples & Analogies
Think about a perfectly balanced see-saw; as one child gets on, another gets off to keep the see-saw level. In the same manner, when heat is applied to the ice, some sticks turn into water (melting), while others are being frozen again by water molecules.
Liquid-Vapour Equilibrium
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This equilibrium can be better understood if we consider the example of a transparent box carrying a U-tube with mercury (manometer). After removing the drying agent by tilting the box on one side, a watch glass (or petri dish) containing water is quickly placed inside the box.
Detailed Explanation
In the experimental setup, water evaporates, increasing the water vapour pressure inside the container until it reaches a constant value at equilibrium. The rate of evaporation equals the rate of condensation, leading to a stable vapour pressure that's dependent on temperature.
Examples & Analogies
Imagine spraying perfume in a sealed room. Initially, the scent is strong, but over time it disperses evenly throughout the room; the rate of scent particles entering and leaving the air becomes constant, similar to how water vapour and liquid water reach equilibrium.
Solid-Vapour Equilibrium
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Let us now consider the systems where solids sublime to vapour phase. If we place solid iodine in a closed vessel, after sometime the vessel gets filled up with violet vapour and the intensity of colour increases with time.
Detailed Explanation
When solid iodine is placed in a closed container, it slowly sublimates into iodine vapour, leading to an increase in the vapour's concentration until a dynamic equilibrium is established between the solid and vapour phases.
Examples & Analogies
Think of sugar sitting at the bottom of a warm cup of tea. Initially, it dissolves quickly, but as saturation occurs, there's no more visible sugar left; it reaches a point where the sugar dissolves and recrystallizes at a balancing rate.
Equilibrium Involving Dissolution
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Solids in liquids: We know from our experience that we can dissolve only a limited amount of salt or sugar in a given amount of water at room temperature.
Detailed Explanation
The concept of saturation occurs when no more solute can dissolve in the solvent. In a saturated solution, the rate of dissolution equals the rate at which the dissolved particles precipitate back out, maintaining a dynamic equilibrium.
Examples & Analogies
Imagine making syrup. You can keep adding sugar to water until it no longer dissolves, forming a thick layer at the bottom. The amount of dissolved sugar balances with the undissolved sugar; this is a saturated solution.
Key Concepts
-
Dynamic Equilibrium: This state is characterized by continuous processes where opposing reactions occur at the same rate. For instance, in a liquid-vapour system, the number of molecules evaporating equals those condensing.
-
Phase Transformation: The section outlines phase changes that result in equilibrium, uncomplicated by temperature or pressure variations. Notably, for water, solid (ice) and liquid coexist at 273K, showcasing equilibrium dynamics.
-
Liquid-Vapour Equilibrium: Discussing vapour pressure, the section illustrates how an increase in temperature raises root pressure, leading to more active evaporation until equilibrium is reached.
-
Solid-Vapour and Solid-Liquid Equilibrium: Examples like sublimation, illustrated by iodine and camphor, demonstrate how solids can transition to gaseous states, emphasizing the balance needed for equilibrium.
-
Dissolution Dynamics: Last but not least, heterogeneous equilibria involving dissolution are covered, cementing the necessity of understanding ionic interactions in solubility and reactions in balanced mixtures.
-
This comprehensive overview is critical not only for theoretical understanding but also for practical applications in chemistry, highlighting the intrinsic relationships that govern physical equilibria.
Examples & Applications
When ice melts at 273K, the amounts of ice and water remain constant despite continuous molecular activity.
A thermometer placed in a water-vapour space shows constant pressure once equilibrium is established.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When vapour and liquid dance, equilibrium takes its stance.
Stories
Imagine a sealed jar of water where some water vapour escapes, while some condenses back — this is how equilibrium works, always balancing itself.
Memory Tools
E-Excel (Evaporation vs. condensation) – remember: Equilibrium is when E-vaporation equals C-ondensation.
Acronyms
D.A.N.C.E - Dynamic, Active, No change, Ever!
Flash Cards
Glossary
- Dynamic Equilibrium
A state in which the rates of forward and reverse processes are equal, resulting in constant concentrations of reactants and products.
- Vapour Pressure
The pressure exerted by the vapour in contact with its liquid or solid form at a given temperature.
Reference links
Supplementary resources to enhance your learning experience.
