Di- and Polybasic Acids and Di- and Polyacidic Bases
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Defining Di- and Polybasic Acids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss di- and polybasic acids. Can anyone tell me what that means?

I think it means acids that can release more than one hydrogen ion.

Exactly! Di- and polybasic acids can donate multiple protons. For example, sulfuric acid can donate two protons. Let's represent that. CO, can someone remind me of the first step in the ionization for a dibasic acid?

H2X produces H+ and HX–.

That's right! And then the second ionization step involves HX- producing another H+ and X2-. Remember these steps when thinking about the strength of an acid.

So, stronger acids will have larger Ka values?

Correct! The larger the Ka, the stronger the acid. Let's keep this in mind as we go deeper into the topic.
Ionization Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's discuss Ka values further. Why do you think they decrease with each successive ionization?

It's probably because as more protons are removed, it gets harder to remove the next one?

Exactly! The remaining ions are negatively charged, making it tougher to release more protons. So when we refer to a dibasic acid, we often represent both it's first and second ionization constants, Ka1 and Ka2. Does anyone have an example of such an acid?

What about phosphoric acid?

Great example! H3PO4 has three ionization constants corresponding to each step of acid dissociation. Keep visualizing these structures!
Application and Importance
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, can someone think of how knowing about these acids is useful in real-world applications?

Maybe in biological systems, where pH levels need to be maintained?

Spot on! Acids play a critical role in maintaining physiological pH levels in organisms. Buffers often involve these acids.

Are there industrial applications too?

Absolutely, many manufacturing processes require acid-base equilibria to control the reactions efficiently. Keep in mind how these processes rely on the principles we've discussed, especially regarding di- and polybasic acids.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Di- and polybasic acids can release more than one proton in solution, leading to multiple ionization steps. This section examines the ionization reactions and constants for these acids, highlighting their importance in chemical equilibrium and acidity.
Detailed
Di- and Polybasic Acids and Di- and Polyacidic Bases
Di- and polybasic acids are acids that can donate two or more protons (H+ ions) respectively. Examples of such acids include oxalic acid, sulfuric acid, and phosphoric acid, each characterized by multiple ionization steps. The ionization of a dibasic acid (H2X) can be represented in two steps:
- First Ionization:
- H2X(aq) ⇌ H+(aq) + HX–(aq)
- The ionization constant (Ka1) for this reaction reflects the strength of the acid.
- Second Ionization:
- HX–(aq) ⇌ H+(aq) + X2–(aq)
- The second ionization constant (Ka2) indicates the dissociation of the first ionized form into its final form, X2–.
This pattern extends to tribasic acids, such as H3PO4, which have three dissociation steps and associated ionization constants. As the order of ionization proceeds, it is common for the ionization constants (Ka) to decrease, indicating a weaker tendency to donate additional protons after the first. When measuring the strength of these acids, it is crucial to also consider the influence of the conjugate bases produced from these successive ionizations, where each subsequent conjugate base becomes progressively stronger.
A fundamental aspect of these acids is how bond strength and the nature of the resultant ions affect the acidity. The strength of the bonds holding the protons influences their release during dissociation, making bond polarity and strength significant factors in analyzing acid strength. The relationships between various acids and their respective bases are crucial for understanding equilibria in chemical reactions, particularly in buffers and acid-base titrations.
Youtube Videos


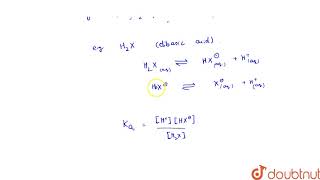






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Polyprotic Acids
Chapter 1 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Some of the acids like oxalic acid, sulphuric acid and phosphoric acids have more than one ionizable proton per molecule of the acid. Such acids are known as polybasic or polyprotic acids.
Detailed Explanation
Polybasic acids, also known as polyprotic acids, are those that can donate more than one proton (H+) per molecule in a solution. Unlike monobasic acids, which release only one H+ ion, polyprotic acids undergo sequential ionization steps, meaning they can donate multiple protons. For example, sulfuric acid (H2SO4) can first lose one proton to become hydrogen sulfate (HSO4–) and then lose another to become sulfate (SO4^2–). This characteristic affects their strength and behavior in chemical reactions.
Examples & Analogies
Think of a multi-layered cake. Just as each layer can be cut separately, polyprotic acids can release their protons one by one, each layer being a step in the overall reaction. For instance, when you bite into a piece of layered cake, you can experience one flavor at a time, analogous to how you would experience the reaction of the acid as it donates one proton after another.
Ionization Reactions and Constants
Chapter 2 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ionization reactions for example for a dibasic acid H2X are represented by the equations:
H2X(aq) H+(aq) + HX–(aq)
HX–(aq) H+(aq) + X2–(aq)
And the corresponding equilibrium constants are given below:
Ka1= {[H+][HX–]} / [H2X] and
Ka2 = {[H+][X2-]} / [HX-]
Detailed Explanation
For a dibasic acid, the ionization occurs in two steps. The first ionization constant, denoted as Ka1, measures the extent to which the acid can donate its first proton. The second ionization constant, Ka2, indicates the extent of the second proton's donation. Each ionization is characterized by an equilibrium constant, showing how favorably the acid dissociates in a given step. The larger the Ka value, the stronger the acid is in that ionization step.
Examples & Analogies
Consider climbing a staircase. The first step may be easier to take than the second, reflecting how some acids can lose their first proton more easily than the second one. Hence, while the first step (first ionization) might be comfortable, the second step (second ionization) could feel more challenging, analogous to the differing ease with which acids release their protons.
Trispecific Acids
Chapter 3 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Similarly, for tribasic acids like H3PO4 we have three ionization constants.
Detailed Explanation
Tribasic acids can donate three protons sequentially, leading to three separate ionization reactions. Each step has its own equilibrium constant: Ka1 for the first proton, Ka2 for the second, and Ka3 for the third. As with dibasic acids, the order in which these protons are lost affects the strength of the acid; generally, the first proton is the easiest to lose.
Examples & Analogies
Imagine a tree with three branches. Each time you try to remove a branch—representing the release of a proton—you'll find it easier to detach the first branch than the others. This analogy mirrors how the first proton of a tribasic acid like phosphoric acid is more readily lost than the second or third, which require relatively more energy or specific conditions to be released.
Strength of H-A Bonds and Acidity
Chapter 4 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In general, when strength of H-A bond decreases, that is, the energy required to break the bond decreases, HA becomes a stronger acid.
Detailed Explanation
The strength of an acid is not only influenced by its ability to release protons but also by the strength of the bond holding those protons. Stronger acids generally have weaker bonds holding the hydrogen atoms, making it easier to donate protons. Therefore, when assessing acidity, one must also consider the bond enthalpy of H-A; weaker bonds correspond with stronger acidic behavior.
Examples & Analogies
Consider breaking a stick—if the stick is thin and fragile, it breaks easily (much like a weak H-A bond). Conversely, a thick and sturdy branch requires much more effort to break (similar to a strong H-A bond). Thus, weak sticks represent strong acids—they can be ‘broken’ easily under the right conditions.
Polarity of H-A Bonds
Chapter 5 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Also, when the H-A bond becomes more polar i.e., the electronegativity difference between the atoms H and A increases and there is marked charge separation, cleavage of the bond becomes easier thereby increasing the acidity.
Detailed Explanation
Polarity in chemical bonds arises due to differences in electronegativity, which can affect an acid's strength. A highly polar H-A bond, where the A is much more electronegative than H, results in increased ionization as the positive H+ ion is more easily released. The greater the difference in electronegativity, the easier it is for the bond to be broken, leading to a stronger acid.
Examples & Analogies
Think of a tug of war between two teams where one team is much stronger (in terms of electronegativity). The stronger team will easily pull over the weaker one (the H atom), similar to how a highly polar bond facilitates the release of the hydrogen ion in acidic solutions.
Size of Atom and Acid Strength
Chapter 6 of 6
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
But it should be noted that while comparing elements in the same group of the periodic table, H-A bond strength is a more important factor in determining acidity than its polar nature. As the size of A increases down the group, H-A bond strength decreases and so the acid strength increases.
Detailed Explanation
When comparing acids within the same group of the periodic table, the size of the atom (A) plays a significant role in acidity. As the atomic radius increases, the bond length between H and A also increases, which reduces bond strength. This means that larger atoms lead to weaker H-A bonds, which in turn makes the acid stronger, as it can release protons more easily.
Examples & Analogies
Imagine trying to hold onto a balloon (representing the H-A bond). As the balloon gets larger (representing a larger A), it becomes harder to hold on to, symbolizing how the bond weakens as atomic size increases, thus depicting the concept of stronger acidity due to longer, weaker bonds.
Key Concepts
-
Di- and Polybasic Acids: Acids capable of donating two or more protons respectively.
-
Ionization Constants: Reflect the strength of the acids, with decreasing values for successive ionizations.
-
Conjugate Bases: Result from the deprotonation of acids and play a crucial role in acid-base equilibria.
Examples & Applications
Example of a dibasic acid: Sulfuric acid (H2SO4) which ionizes to release two protons.
Phosphoric acid (H3PO4) demonstrates a triprotic system with three ionization steps.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Dibasic acids donate two lots, strong like chains that hold many knots.
Stories
Imagine a curious octopus in a lab who can give away two of its arms to participate in experiments; this is like a dibasic acid donating its protons.
Memory Tools
DAB - DiBasic Acid Bonds (reminds about the two protons).
Acronyms
DPA for Dibasic Polyprotic Acids.
Flash Cards
Glossary
- Dibasic Acid
An acid that can donate two protons to bases.
- Polybasic Acid
An acid that can donate more than two protons.
- Ionization Constant
The equilibrium constant for the ionization of an acid in water.
- Ka
The acid dissociation constant; a measure of the strength of an acid in solution.
- Conjugate Base
The species that remains after an acid has donated a proton.
Reference links
Supplementary resources to enhance your learning experience.
