Common Ion Effect on Solubility of Ionic Salts
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Common Ion Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're going to discuss the common ion effect. Does anyone know what we mean by 'common ion'?

Is it an ion that is present in more than one compound in solution?

Exactly! A common ion is an ion that is already present in the solution due to the presence of another salt. Now, how does adding a common ion affect the solubility of a salt?

I think it decreases the solubility, right?

Right! This relates to Le Chatelier's principle, which states that a system at equilibrium will adjust to counteract any change. Let’s consider an example to visualize this effect.

Is the example you have the one with AgCl and NaCl?

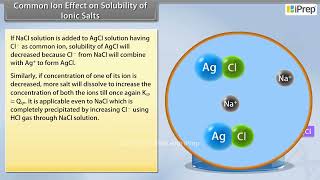
Yes! When AgCl is dissolved, it dissociates into Ag+ and Cl− ions. If we add NaCl to this solution, which also provides Cl− ions, what happens to the AgCl?

The solubility will decrease since more Cl− ions will push the equilibrium to the left.

Exactly! This is the common ion effect in action. Great participation, everyone!
Chemical Equilibria and Common Ion Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let's delve deeper into how the common ion effect changes the equilibrium expression. How do we express Ksp, the solubility product constant?

Isn’t it the product of the concentrations of the ions in solution?

Right! For AgCl, the Ksp expression is Ksp = [Ag+][Cl−]. When we add a common ion like Cl−, what happens to the concentrations in the equilibrium expression?

The concentration of Cl− increases, which will affect the net concentrations of Ag+.

Exactly! The increase in [Cl−] results in a decrease in [Ag+] at equilibrium, demonstrating how common ions can shift equilibrium.

So this means that in analytical applications, if you want to precipitate a certain ion, you can use a common ion?

That's right! It’s widely used in quantitative chemical analysis to precipitate specific ions. Let's summarize what we've learned.

In this session, we covered how adding a common ion affects solubility, concentration, and the Ksp expression.
Applications of Common Ion Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's talk about real-life applications of the common ion effect. Can someone think of a scenario where this might be useful?

Maybe in water treatment?

Absolutely! Understanding how to manipulate ion concentrations helps us in water softening and removing unwanted ions. Any other scenarios?

In the laboratory for gravimetric analysis?

Yes! By adding a common ion, we can selectively precipitate ions for quantitative analysis, ensuring higher purity. Remember, the key takeaway is to control concentrations effectively.

Is there a specific example of how this is applied?

Sure! For example, adding NaCl to a solution containing silver ions will precipitate silver chloride. Excellent discussion today, everyone!

In summary, understanding the common ion effect allows for practical applications in both industrial and laboratory settings.
Understanding the Mechanism of Common Ion Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

To further understand the common ion effect, let’s discuss the underlying mechanisms. How does the presence of a common ion affect solubility precipitates?

It essentially pushes the equilibrium in the opposite direction, right?

Yes, it causes precipitation to occur until the state of equilibrium is reestablished. This is a direct application of Le Chatelier’s principle.

So, this means that the stronger the common ion effect, the more salt can be precipitated?

Exactly! The degree of precipitation depends on the concentration of the common ion. If the ion concentration is large enough, a nearly complete precipitation can occur.

Let's recap: today we discussed how the common ion effect operates, its mechanisms, and its importance in solution chemistry.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section discusses the common ion effect and its influence on the solubility of ionic salts. It explains how the presence of a common ion decreases the solubility of a salt by shifting the equilibrium, illustrating this principle with practical applications in precipitation and solubility product constants.
Detailed
In the context of Le Chatelier’s principle, the common ion effect illustrates that the solubility of an ionic salt is reduced when a common ion is added to a solution. For instance, if we consider a saturated solution of a salt such as silver chloride (AgCl), the equilibrium can be described as AgCl(s) ↔ Ag+(aq) + Cl−(aq). If a salt like NaCl, which dissociates to provide Cl−, is added, it increases the concentration of Cl− ions. As a result, the system responds by shifting the equilibrium to the left, leading to decreased solubility of AgCl and the precipitation of solid AgCl. This principle has significant implications in analytical chemistry and industrial processes, where precise control over ion concentrations is required.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Common Ion Effect
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is expected from Le Chatelier’s principle that if we increase the concentration of any one of the ions, it should combine with the ion of its opposite charge and some of the salt will be precipitated till once again Ksp = Qsp.
Detailed Explanation
Le Chatelier’s principle states that if a system at equilibrium is disturbed, the system will adjust itself to counteract the disturbance and restore equilibrium. When the concentration of one of the ions is raised, the equilibrium shifts in a way that reduces this disturbance, usually by precipitating some of the salt out of solution. This helps maintain the solubility product constant (Ksp) at a steady value.
Examples & Analogies
Think of a crowded room where people are trying to move around. If you add more people (increase the concentration of an ion), the movement will be restricted, and some people might have to leave the room temporarily so that the remaining people can find space. In this analogy, those who leave represent the precipitated salt.
Precipitation and Solubility Dynamics
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Similarly, if the concentration of one of the ions is decreased, more salt will dissolve to increase the concentration of both the ions till once again Ksp = Qsp.
Detailed Explanation
If the concentration of a particular ion in a saturated solution is reduced, the equilibrium responds by dissolving more of the solid salt to increase the concentration of the ions back toward the established Ksp value. This dynamic means that the system constantly shifts to maintain equilibrium.
Examples & Analogies
Imagine a tank filled with water (the saturated solution) having a filter (the solid salt). If you remove some water (decrease an ion's concentration), the filter will start to dissolve more to refill the tank back to its original level. This is how maintaining equilibrium works in solutions.
Common Ion Effect in Precipitation Reactions
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The common ion effect is also used for almost complete precipitation of a particular ion as its sparingly soluble salt, with very low value of solubility product for gravimetric estimation.
Detailed Explanation
In analytical chemistry, the common ion effect is utilized to precipitate specific ions from solution, making them easier to measure. For instance, when a highly soluble salt and a sparingly soluble salt share a common ion, the addition of the common ion can drive the equilibrium towards precipitation of the sparingly soluble salt.
Examples & Analogies
Think of baking soda (sodium bicarbonate) which can help separate oil from water in a salad dressing. By adding an ingredient that creates a common link (baking soda for instance), you promote the aggregation (or precipitation) of particles that need to be removed or measured.
Practical Application of Common Ion Effect
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Thus we can precipitate silver ion as silver chloride, ferric ion as its hydroxide (or hydrated ferric oxide) and barium ion as its sulphate for quantitative estimations.
Detailed Explanation
In practical applications, certain salts can be precipitated from solutions when a common ion is added. For example, adding sodium chloride (providing Cl– ions) to a solution containing Ag+ ions causes silver chloride (AgCl) to precipitate out because Ksp for AgCl is low. This principle is pivotal in quantitative analysis where it aids in estimating the concentration of ions in solution.
Examples & Analogies
Consider a situation where you want to extract gold from a mixture. By adding substances that contain ions that bond with the impurities, you help precipitate them out, leaving behind purer gold. This reflects how common ion usage in chemistry can help achieve desired purifications!
Example Calculation of Molar Solubility
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
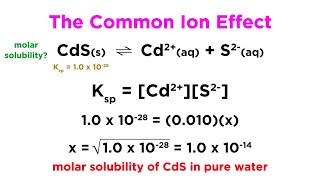
The dissolution of S mol/L of Ni(OH)2 provides S mol/L of Ni2+ and 2S mol/L of OH–, but the total concentration of OH– = (0.10 + 2S) mol/L because the solution already contains 0.10 mol/L of OH– from NaOH.
Detailed Explanation
When calculating the molar solubility of sparingly soluble salts, you need to take into account existing concentrations of common ions already present in the solution. For Nickel(II) hydroxide, the presence of OH– from sodium hydroxide alters the typical solubility equation, leading to necessary adjustments in calculations to find a new equilibrium concentration.
Examples & Analogies
It's similar to adding sugar to water that already has some sugar in it. You can't just count the additional amount of sugar you're adding (or dissolving); you must consider how much sugar is already there, which changes the final sweetness of the solution.
Key Concepts
-
Common Ion Effect: The decrease in solubility of a salt when a common ion is added to the solution.
-
Ksp: An equilibrium constant specific to the solubility of ionic compounds in saturated solution.
-
Le Chatelier’s Principle: A principle stating that a system at equilibrium will adjust to counteract applied changes.
Examples & Applications
Adding NaCl to a saturated AgCl solution decreases the solubility of AgCl because the increase in Cl− ion concentration shifts the equilibrium to the left.
When a solution of calcium hydroxide is saturated, adding sodium hydroxide increases the concentration of OH− ions, leading to the precipitation of more calcium hydroxide.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
When common ion is near, solubility fears, it drops down low, as the equilibrium flows.
Stories
Imagine a dance floor where too many of one partner (the common ion) make it hard for everyone to dance (dissolve).
Memory Tools
Common Ions Can Cause Solubility Collapse (CI-C2S).
Acronyms
CIE - Common Ion Effect.
Flash Cards
Glossary
- Common Ion Effect
The phenomenon where the solubility of an ionic compound decreases when a common ion is added to the solution.
- Solubility Product Constant (Ksp)
An equilibrium constant that applies to the solubility of sparingly soluble ionic compounds.
- Le Chatelier’s Principle
A principle stating that if an external change is applied to a system at equilibrium, the system will adjust to counteract that change.
Reference links
Supplementary resources to enhance your learning experience.
