EQUILIBRIUM IN CHEMICAL PROCESSES – DYNAMIC EQUILIBRIUM
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Dynamic Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're exploring dynamic equilibrium in chemical processes. It's defined as the state where the forward and reverse reactions occur at the same rate, leading to stable concentrations of reactants and products. Can one of you provide an example of this?

What about the evaporation and condensation of water?

Exactly! In a closed container, water molecules evaporate and condense at equal rates. Remember, this process demonstrates equilibrium. Let's dive deeper into the characteristics of dynamic equilibrium. What do you think happens to the concentrations at this stage?

They must stay constant, right?

Correct! Continuous reactions keep moving without changing the concentrations. That’s what makes equilibrium dynamic. So, can we classify equilibria into different types based on their reactions?

Maybe by whether they go to completion or have substantial amounts of both reactants and products?

Great thinking! There are indeed classifications based on the extent of reaction and concentration of species involved. Let’s remember this framework of dynamic behavior in future discussions.
Le Chatelier's Principle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s talk about how changes in conditions affect our equilibrium state through Le Chatelier's Principle. When we add more reactants, what can you predict will happen?

The equilibrium shifts towards producing more products, right?

Exactly! The system compensates for the added reactant to restore equilibrium. What other factors might affect dynamic equilibrium?

I think pressure changes can also impact it, especially for gaseous reactions.

Correct! Increasing pressure favors the side of the reaction with fewer moles of gas. Remember this when analyzing reactions under varying conditions. Now let’s discuss catalysts. How do they interact with equilibrium?

They don't change the equilibrium position but speed up the reaction to reach equilibrium faster.

Exactly right! Catalysts do not affect the equilibrium position; they only hasten the response to reach that equilibrium state.
Understanding Equilibrium Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s shift gears to equilibrium constants, denoted as Kc. They help us describe the balance between reactants and products quantitatively. Who can tell me what Kc represents?

It's the ratio of product concentrations to reactant concentrations, each raised to the power of their coefficients in the balanced equation.

Exactly! For any reaction like A + B ⇌ C + D, the expression is Kc = [C][D]/[A][B]. What about changes in temperature? How does it affect Kc?

Kc changes with temperature, and its value indicates if the reaction is exothermic or endothermic.

Right! In an exothermic reaction, increasing temperature decreases Kc, while in endothermic reactions, it increases Kc. Let’s summarize these concepts we discussed about dynamic equilibria and their significance.

We learned how equilibrium is dynamic, how conditions affect the state, and how equilibrium constants work!

Wonderful recap! Understanding these concepts will strengthen our grasp of responses in chemical processes.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
Dynamic equilibrium in chemical processes entails that while reactions occur in both directions, the rates at which products form and reactants revert are equal, leading to stable concentrations. This section explores characteristics of equilibrium, the factors influencing it, and the significance of equilibrium constants.
Detailed
Dynamic Equilibrium in Chemical Processes
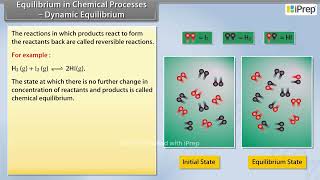
Dynamic equilibrium is a state in which both the forward and reverse reactions occur at equal rates in a chemical reaction, leading to a constant concentration of reactants and products over time. The concept hinges on the fact that reactions are not static; rather, they engage in continuous activity, suggesting movement even in equilibrium. This equilibrium can be established in both physical processes, such as evaporation and condensation of liquids, and in chemical reactions involving several species.
Characteristics of Dynamic Equilibrium
- Reversible Reactions: Dynamic equilibrium only occurs with reversible reactions. Both the forward and reverse processes operate concurrently.
- Equal Rates: At equilibrium, the rate of formation of products equals the rate of formation of reactants.
- Constant Concentrations: While both reactants and products are present in reactions, their concentrations remain constant.
- Equilibrium Mixture: The composition at equilibrium is referred to as the equilibrium mixture.
Equilibrium states are governed by external conditions, such as concentration, temperature, pressure, and the presence of catalysts, aligning with Le Chatelier’s Principle. This principle posits that any change in these factors prompts the system to readjust to minimize the effect of that change. Consequently, altering concentration or pressure can shift equilibrium toward products or reactants, affecting the yield of desired chemicals in both laboratory and industrial processes. Moreover, equilibrium constants (Kc) quantify the relationship between concentrations of reactants and products, allowing scientists to predict the extent of chemical reactions under specific conditions.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Dynamic Equilibrium
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Analogous to the physical systems, chemical reactions also attain a state of equilibrium. These reactions can occur both in forward and backward directions. When the rates of the forward and reverse reactions become equal, the concentrations of the reactants and the products remain constant. This is the stage of chemical equilibrium. This equilibrium is dynamic in nature as it consists of a forward reaction in which the reactants give product(s) and reverse reaction in which product(s) gives the original reactants.
Detailed Explanation
Dynamic equilibrium is a state where two opposing processes occur at the same rate, resulting in no net change in concentration of reactants and products. This means that while the reactants are turning into products, some of those products are also converting back into reactants, maintaining constant concentrations over time.
Examples & Analogies
Think of dynamic equilibrium like a busy train station. Trains (reactants) arrive to pick up passengers (products) while simultaneously, some passengers leave to board a train heading back to their original station. Although the number of people at the station may seem constant, there is always movement happening.
Visualization of Chemical Equilibrium
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For a better comprehension, let us consider a general case of a reversible reaction, A + B ⇌ C + D. With passage of time, there is accumulation of the products C and D and depletion of the reactants A and B. This leads to a decrease in the rate of forward reaction and an increase in the rate of the reverse reaction.
Detailed Explanation
In the reaction A + B ⇌ C + D, as reactants A and B are consumed to form products C and D, the rate of formation of products increases. At the same time, products C and D are beginning to convert back into reactants, which causes their concentrations to decrease. Eventually, the rates of the two reactions will balance out, leading to a stable concentration of all species involved.
Examples & Analogies
Imagine a seesaw. As more weight is added to one side (forming products), it moves downward. However, as some of that weight (products) is removed to the other side (as reactants), the seesaw begins to balance. Once it’s balanced, the weight on both sides remains constant, representing a dynamic equilibrium.
Application: Haber Process
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The dynamic nature of chemical equilibrium can be demonstrated in the synthesis of ammonia by Haber’s process. In a series of experiments, Haber started with known amounts of dinitrogen and dihydrogen maintained at high temperature and pressure and at regular intervals determined the amount of ammonia present. After a certain time, the composition of the mixture remains the same even though some of the reactants are still present.
Detailed Explanation
In the Haber process, nitrogen (N2) and hydrogen (H2) gases react to form ammonia (NH3). Over time, as the reaction proceeds, more ammonia is formed until a point where the rates of formation of ammonia and its decomposition back to nitrogen and hydrogen become equal. This means that even if the reaction still occurs, the overall amounts of reactants and products do not change, exemplifying dynamic equilibrium.
Examples & Analogies
Consider a bathtub filling with water while a drain is always open. If you fill the tub at the same rate that water is draining out, the water level stays constant. This is similar to the dynamic equilibrium in chemical reactions where the formation and decomposition of substances continue at equal rates.
Other Reactions and Equilibria
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Similarly, the reaction can reach the state of equilibrium even if we start with only C and D; that is, no A and B being present initially, as the equilibrium can be reached from either direction.
Detailed Explanation
In reversible reactions, the state of equilibrium can be achieved regardless of whether reactants or products are initially present. If we start with products C and D, the system will still shift to form reactants A and B until balance is reached. This showcases the flexibility and symmetry in dynamic equilibrium.
Examples & Analogies
Imagine a crowd at a concert. It can start with people already inside the venue (products) or those still outside trying to get in (reactants). Eventually, regardless of where they start, the crowd can stabilize at a certain number, reflecting the dynamic nature of reaching equilibrium in social situations.
Key Concepts
-
Reversible reactions reach dynamic equilibrium when reactants and products are in constant concentrations.
-
Le Chatelier's Principle indicates how equilibrium shifts in response to changes in concentration, pressure, and temperature.
-
Equilibrium constants, such as Kc and Kp, provide quantitative measures of the extent of reactions at equilibrium.
Examples & Applications
The evaporating water in a closed container reaches a dynamic equilibrium when the rate of evaporation equals the rate of condensation.
In the reaction of hydrogen and iodine, starting from either side leads to the same concentration of HI at equilibrium.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
At equilibrium, reactions flow, forward and back, it’s the show.
Stories
Imagine a busy train station where trains arrive and depart at the same rate, representing the dynamic equilibrium of reactions.
Memory Tools
Remember Kc: Keep Reactants on the left, Products to the right!
Acronyms
LET (Le Chatelier's Principle, Equilibrium, Temperature) - to remember how changes affect equilibrium.
Flash Cards
Glossary
- Dynamic Equilibrium
A state in which the rates of forward and reverse reactions are equal, resulting in constant concentrations of reactants and products.
- Equilibrium Constant (Kc)
A numerical value that expresses the ratio of the concentrations of products to reactants at equilibrium.
- Le Chatelier's Principle
A principle stating that if an external change is applied to a system at equilibrium, the system adjusts to minimize that change.
Reference links
Supplementary resources to enhance your learning experience.
