Equilibrium Constant in Gaseous Systems
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Kc and Kp
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we will be discussing the equilibrium constant, K, specifically Kc for concentrations and Kp for partial pressures. Can someone remind me what an equilibrium constant represents?

It shows the ratio of the products' concentrations to the reactants' concentrations at equilibrium.

Exactly! For a reaction like aA + bB ⇌ cC + dD, the equilibrium constant Kc is expressed as Kc = [C]^c[D]^d / [A]^a[B]^b. Remember that the concentrations are raised to the power of their coefficients.

And Kp is similar but uses the partial pressures instead, right?

Absolutely! Kp = (P_C)^c(P_D)^d / (P_A)^a(P_B)^b. It's often easier to use Kp for gaseous reactions because pressure directly correlates with the number of moles in a gas.

How do we relate Kp and Kc?

Good question! The relationship is expressed as Kp = Kc (RT)^{Δn}, where Δn is the change in moles of gaseous products minus the moles of gaseous reactants. Remember that R is the gas constant and T is the temperature.
Le Chatelier's Principle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's discuss Le Chatelier's Principle. Who can explain how it relates to chemical equilibrium?

It says that if a change is applied to an equilibrium system, the system will adjust to counteract that change.

Exactly! For instance, if we increase the pressure in a gaseous reaction, which way does the equilibrium shift?

It shifts towards the side with fewer moles of gas.

Correct! Similarly, what happens if we increase temperature in an exothermic reaction?

The equilibrium shifts towards the reactants, right?

Yes, very good! Understanding this principle helps us control reactions in industrial processes.
Applications of Equilibrium Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s explore how we can use the equilibrium constants to predict reaction behavior. If we know Kp for a reaction, what can we derive?

We can determine the partial pressures at equilibrium!

Right! And if Qp is different from Kp, what does that tell us about the reaction?

It tells us if the reaction is at equilibrium or if it will shift towards products or reactants.

Exactly! If Qp < Kp, the reaction shifts towards products; if Qp > Kp, it shifts towards reactants.

Can we also calculate changes in concentrations from this information?

Definitely! Understanding how to manipulate Kc and Kp allows chemists to optimize conditions for desired outcomes.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The equilibrium constant (Kp) for gaseous systems is typically expressed in terms of partial pressures, while Kc is based on molar concentrations. The section outlines how changes in conditions affect equilibriums and introduces the relationship between Kp and Kc. Additionally, it explains how equilibrium can be reached from different starting conditions.
Detailed
Equilibrium Constant in Gaseous Systems
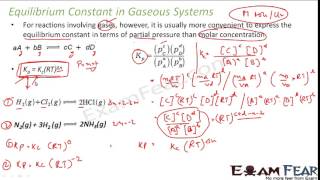
This section focuses on the equilibrium constant, which is a fundamental concept in chemical equilibrium, particularly for gaseous reactions. For a reaction represented by the general equation:
$$ aA + bB \rightleftharpoons cC + dD $$
the equilibrium constant (Kc) is expressed in terms of the molar concentrations of the reactants and products at equilibrium:
$$ K_c = \frac{[C]^c[D]^d}{[A]^a[B]^b} $$
Where [A], [B], [C], and [D] are the equilibrium concentrations of each species. For reactions involving gases, it is often more convenient to use partial pressures, represented as Kp:
$$ K_p = \frac{(P_C)^c(P_D)^d}{(P_A)^a(P_B)^b} $$
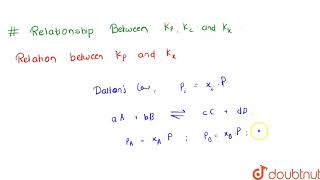
Here, P represents the partial pressures of the gases involved. The relationship between Kp and Kc can be summarized as:
$$ K_p = K_c (RT)^{\Delta n} $$
where Δn is the change in moles of gas during the reaction. If there are no changes in moles, Kp and Kc are numerically equal under standard conditions.
Changes in concentration, pressure, and temperature can shift the equilibrium position of reactions, as explained by Le Chatelier's principle. This principle states that if an external change is applied to a system at equilibrium, the system will adjust to counteract that change. For example, increasing the pressure in a gaseous reaction favors the direction that produces fewer moles of gas. Conversely, increasing temperature favors endothermic reactions, shifting the equilibrium to the products' side.
Consequently, understanding the equilibrium constant allows chemists to predict the behavior of reactions under varying conditions and to optimize conditions for desired outcomes in industrial applications.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Equilibrium Constants in Gaseous Reactions
Chapter 1 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For reactions involving gases, it is usually more convenient to express the equilibrium constant in terms of partial pressure.
Detailed Explanation
In gaseous systems, instead of using concentrations (Kc), we often use partial pressures (Kp) to express equilibrium constants. This is useful because partial pressure is directly related to the number of gas molecules present in a given volume, making it a practical measure in reactions where gases are involved.
Examples & Analogies
Consider a balloon filled with air. The pressure inside the balloon is influenced by how many air molecules are present. If you were to let a little air out, the pressure would decrease because there are fewer gas molecules. Similarly, in a chemical reaction where gases are involved, the pressure can give us a direct understanding of how the reaction is proceeding.
Ideal Gas Equation and its Relationship with Concentration
Chapter 2 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The ideal gas equation is given as pV = nRT, which can be rearranged to show that the concentration of a gas is proportional to its pressure.
Detailed Explanation
The Ideal Gas Law, pV = nRT, connects pressure (p), volume (V), quantity of gas (n), and temperature (T). By rearranging this, we can express the concentration as n/V = p/RT, showing that the concentration of a gas is proportional to its pressure, especially when temperature is constant. This allows us to convert between concentration and pressure in equilibrium expressions.
Examples & Analogies
Think of a sealed container of carbonated drink. When the cap is on, the gas (carbon dioxide) has a high pressure due to the bottled environment. When you open it, the pressure decreases, leading to gas escaping. The relationship between concentration and pressure is crucial in understanding how much gas can dissolve before reaching equilibrium.
Equilibrium Constant Expressions
Chapter 3 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For the equilibrium H2(g) + I2(g) ⇌ 2HI(g), we can express Kp in terms of partial pressures: Kp = (p_HI)^2 / (p_H2 * p_I2).
Detailed Explanation
For a gaseous reaction, the equilibrium constant Kp can be expressed using the partial pressures of the gases involved. In the case of the reaction between hydrogen and iodine to form hydrogen iodide, the equilibrium expression reflects how the pressure of each gas relates to each other at equilibrium. This is calculated by taking the product of the pressures of the products raised to their stoichiometric coefficients, divided by the reactants' pressures also raised to their coefficients.
Examples & Analogies
Imagine a recipe for making a dish where the right proportions of ingredients are crucial. The balance of the pressure of each gas in a reaction is similar. Too much of one reactant (like hydrogen) would shift the balance and influence how much product (hydrogen iodide) can be formed, just like overdoing one ingredient can ruin a dish.
Comparison of Kp and Kc
Chapter 4 of 4
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Kp and Kc can be related through the equation Kp = Kc (RT)^(Δn), where Δn is the change in moles of gas.
Detailed Explanation
The relationship between Kp and Kc is significant in gaseous equilibria because it highlights that the value of the equilibrium constant will depend not only on the concentrations but also on the temperature and the change in the number of moles of gas in the reaction. Δn represents the difference in the number of moles of gaseous products and reactants: if you have more products than reactants, Δn is positive, and vice versa.
Examples & Analogies
Think of a crowded subway train. If people start getting off at a stop, the 'pressure' or congestion of people decreases. If more people get on, the congestion increases. Similarly, in a chemical equilibrium, the balance of gas molecules influences the relationship between Kp and Kc depending on if more reactants or products are 'fitting' in.
Key Concepts
-
Equilibrium Constant (K): Ratio of concentrations or pressures at equilibrium.
-
Kp vs Kc: Kp applies to gases using partial pressures, while Kc applies to concentrations.
-
Le Chatelier's Principle: Describes how a system at equilibrium reacts to changes.
-
Delta n (Δn): Indicates the change in moles of gas during a reaction.
Examples & Applications
For the reaction 2NO2(g) ⇌ N2O4(g), if the concentration of NO2 is 0.5 M, the equilibrium constant can be calculated using the concentrations of each species at equilibrium.
In a gaseous reaction involving hydrogen and iodine, Kc can be used to determine the formation of HI.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Kc and Kp, a ratio we see, keeps reactions in harmony, like nature's decree.
Stories
Imagine a seesaw with products on one side and reactants on the other; K keeps them balanced as you add weight on either side.
Memory Tools
K for Keep, C for Concentrations, P for Pressures - remember Kc gives ratios for concentrations while Kp is for pressures.
Acronyms
RAP - Reactants Adjust Producing
When conditions change
reactions adjust to maintain equilibrium.
Flash Cards
Glossary
- Equilibrium Constant (K)
A value that expresses the ratio of concentrations of products to reactants at equilibrium.
- Kc
Equilibrium constant based on concentrations of reactants and products in a solution.
- Kp
Equilibrium constant based on the partial pressures of gaseous reactants and products.
- Le Chatelier's Principle
A principle stating that a system at equilibrium will adjust to counteract any changes imposed on it.
- Delta n (Δn)
The difference in the number of moles of gaseous products and reactants.
Reference links
Supplementary resources to enhance your learning experience.
