Liquid-Vapour Equilibrium
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Liquid-Vapour Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're exploring liquid-vapour equilibrium. Can anyone explain what happens when you put water in a closed container?

I think the water will start to evaporate until something happens.

Exactly! The water molecules escape into the vapour phase while some return to the liquid. This process continues until the rate of evaporation equals the rate of condensation, creating a dynamic equilibrium.

So the pressure in the container stays the same?

Correct! At equilibrium, the vapour pressure remains constant at a specific temperature, known as the equilibrium vapour pressure.

Let’s remember: 'Evaporation and condensation are like a dance at equilibrium!'

Can we relate the temperature to the equilibrium pressure?

Yes! Higher temperatures usually increase vapour pressure. Higher kinetic energy means more molecules can escape from the liquid.
Characteristics of Liquid-Vapour Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, can anyone describe the dynamic nature of equilibria?

It means that while nothing appears to change, there’s actually a lot happening at the molecular level.

Well put! It truly illustrates how equilibrium is constantly active. It’s important to remember that equilibrium can be influenced by factors we will discuss later, such as concentration changes.

And pressure? Does that modify the equilibrium state too?

Absolutely! Changes in pressure can affect where the equilibrium lies, particularly in reactions with different numbers of gaseous molecules.

Let's use the acronym 'LVP' for 'Liquid-Vapour Pressure' to reinforce that equilibrium characteristics apply to liquid-vapour systems.
Examples of Liquid-Vapour Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Can anyone provide an example of liquid-vapour equilibrium?

What about the boiling of water in a kettle?

Great example! When water boils, it reaches a point where the vapour pressure equals atmospheric pressure, leading to rapid evaporation.

And what about other liquids? You mentioned they have different vapour pressures, right?

Yes, different liquids will have unique equilibrium vapour pressures. For instance, ethanol has a higher vapour pressure than water at the same temperature, indicating it's more volatile.

Remember the phrase 'Higher temperature, higher vapour pressure' to help recall the relationship between temperature and vapour pressure!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section elaborates on liquid-vapour equilibrium through various examples, emphasizing how the dynamic nature of the system leads to a consistent vapour pressure. It also details the effect of different substances on equilibrium states and the conditions under which equilibrium is established.
Detailed
Liquid-Vapour Equilibrium
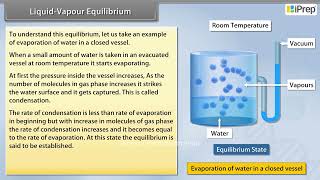
In the study of chemical equilibria, the liquid-vapour equilibrium is a crucial aspect. When a liquid is placed in a closed container, it evaporates, forming vapour. Molecules with higher kinetic energy escape the liquid phase, while some vapour molecules return to the liquid, leading to a state where the rates of evaporation and condensation balance each other. This condition is termed dynamic equilibrium.
At this point, the quantity of vapour pressure remains constant at a specific temperature, known as the equilibrium vapour pressure. Various substances exhibit different equilibrium vapour pressures at the same temperature, giving insight into their volatility and boiling points. For example, water reaches its boiling point at 100°C and a pressure of 1.013 bar, while changes in altitude can affect this boiling point.
Dynamic equilibrium reflects the continuous exchange of molecules between phases, underlining that systems at equilibrium exhibit constant macroscopic properties despite ongoing microscopic activities. Furthermore, the section discusses how both the physical state of substances and chemical reactions can attain equilibrium, depending on the concentration, temperature, or pressure conditions applied. These principles serve essential applications in both industrial processes and laboratory settings.
Youtube Videos







Audio Book
Dive deep into the subject with an immersive audiobook experience.
Introduction to Liquid-Vapour Equilibrium
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This equilibrium can be better understood if we consider the example of a transparent box carrying a U-tube with mercury (manometer). Drying agent like anhydrous calcium chloride (or phosphorus penta-oxide) is placed for a few hours in the box. After removing the drying agent by tilting the box on one side, a watch glass (or petri dish) containing water is quickly placed inside the box. It will be observed that the mercury level in the right limb of the manometer slowly increases and finally attains a constant value, that is, the pressure inside the box increases and reaches a constant value. Also the volume of water in the watch glass decreases.
Detailed Explanation
In a closed system, when a drying agent is used to remove moisture, its removal creates a vacuum. When water is introduced, it begins to evaporate. Initially, there are very few water molecules in the vapor phase, leading to an increase in vapor pressure. Over time, as more water evaporates, the pressure continues to rise until it stabilizes. This indicates that the system has reached a state of equilibrium where the pressure exerted by water vapor remains constant.
Examples & Analogies
Imagine placing a lid on a pot of water. As the water heats up, steam rises, but as more steam fills the pot, it can condense back into liquid water. Eventually, the amount of water evaporating equals the amount condensing, demonstrating this equilibrium between liquid and vapor.
Equilibrium Condition
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Initially there was no water vapour (or very less) inside the box. As water evaporated the pressure in the box increased due to addition of water molecules into the gaseous phase inside the box. The rate of evaporation is constant. However, the rate of increase in pressure decreases with time due to condensation of vapour into water. Finally it leads to an equilibrium condition when there is no net evaporation. This implies that the number of water molecules from the gaseous state into the liquid state also increases till the equilibrium is attained i.e., rate of evaporation= rate of condensation.
Detailed Explanation
The key point in this chunk is the focus on equilibrium within the context of evaporation and condensation. As water evaporates, it increases the amount of vapor present, leading to a buildup of pressure. However, as the pressure builds up, it encourages more condensation and thus counters further evaporation. At equilibrium, the rate of water evaporating equals the rate vapor molecules condense back, hence no net change in the amount of water present in either phase.
Examples & Analogies
Think of a sponge soaking up a liquid. Initially, when you dip it in water, it absorbs a lot of liquid. As it gets fuller, it will absorb less and the water remaining will eventually find stability. This is like reaching a point where the sponge isn't soaking up more liquid than it's losing, just like water in equilibrium between its liquid and vapor phases.
Vapour Pressure
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At equilibrium, the pressure exerted by the water molecules at a given temperature remains constant and is called the equilibrium vapour pressure of water (or just vapour pressure of water); vapour pressure of water increases with temperature. If the above experiment is repeated with methyl alcohol, acetone and ether, it is observed that different liquids have different equilibrium vapour pressures at the same temperature, and the liquid which has a higher vapour pressure is more volatile and has a lower boiling point.
Detailed Explanation
The concept of vapor pressure is crucial for understanding the behavior of different liquids. At equilibrium, the vapor pressure is constant and depends on temperature; as temperature increases, more liquid molecules will have enough energy to break free from the liquid into the vapor phase, resulting in a higher vapor pressure. Different substances exhibit different vapor pressures due to variations in their molecular structure and the forces holding them together. For example, a substance with weak intermolecular forces will evaporate more readily than one with strong forces, indicating it is more volatile.
Examples & Analogies
Consider how some scents evaporate quickly while others linger. A light cologne evaporates rapidly into the air (high vapor pressure), while a thick oil takes longer to do so (lower vapor pressure). The rate at which these substances evaporate is closely tied to their respective vapor pressures.
Key Concepts
-
Equilibrium: The state in which the rates of the forward and reverse reactions are equal.
-
Vapour Pressure: The pressure exerted by a vapour in equilibrium with its liquid.
-
Dynamic Nature: Equilibrium is characterized by continuous exchange, making it a dynamic process.
Examples & Applications
A sealed bottle of water at room temperature eventually reaches a consistent vapour pressure when the rates of evaporation and condensation balance.
Different liquids like acetone and ethanol have varying equilibrium vapour pressures, indicating their volatility.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
In a tube, liquid's vapour looms, equilibrium fills all rooms.
Stories
Imagine a busy dance floor, where every dancer is perfectly matched, spinning between the walls of vapour and liquid, always keeping the pace just right.
Memory Tools
LEVA: Liquid Evaporation Vapour Activity, to remember how vapours behave.
Acronyms
DYNAMIC
Defining Your New Activity in Molecular Interactions Continuously.
Flash Cards
Glossary
- Dynamic Equilibrium
A state of balance in a system where opposing processes occur at equal rates.
- Vapour Pressure
The pressure exerted by a vapour in equilibrium with its liquid at a given temperature.
- Equilibrium Vapour Pressure
The vapour pressure measured when a liquid's evaporation equals its condensation in a closed system.
Reference links
Supplementary resources to enhance your learning experience.
