Lewis Acids and Bases
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Lewis Acids and Bases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing an important aspect of acid-base chemistry known as the Lewis theory. According to G.N. Lewis, can anyone tell me how he defines an acid and a base?

An acid is a substance that accepts protons, right?

That's a common definition, but in the Lewis theory, an acid is defined as any species that accepts an electron pair. What about bases?

A base donates electron pairs?

Exactly! So, acid-base reactions can occur even without protons being transferred. Let’s remember this with the acronym 'A for Acceptor' and 'B for Donor', referring to acids and bases, respectively.

Can you give us an example?

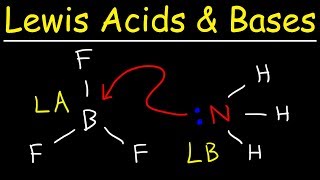
Sure! A classic example is the reaction between boron trifluoride (BF3) and ammonia (NH3), where BF3 accepts an electron pair from NH3.

Remember, BF3 does not contain protons, yet it acts as an acid. Let's summarize: Lewis acids receive electron pairs, whereas bases donate them!
Examining Lewis Acids
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand the basic definitions, let’s explore some common Lewis acids. Who can share examples of species that act as Lewis acids?

I think aluminum chloride and magnesium ions can act as Lewis acids.

Correct! These species can accept electron pairs. What is important to note about these species?

They’re electron deficient?

Exactly! The electron deficiency is what allows them to accept electrons. Remember, they may not always have protons. When we say 'Lewis acids', think about electron pairs rather than protons. It helps differentiate from other definitions.
Examining Lewis Bases
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let’s shift our focus to Lewis bases. Who can tell me what a Lewis base is?

It donates electron pairs.

Correct! Substances like water (H2O), ammonia (NH3), and hydroxide ions (OH–) can act as Lewis bases because they have lone pairs to donate. Why is it useful to know this?

It helps us understand reactions that don't involve protons.

Exactly! The Lewis concept is broader than traditional definitions and allows us to understand many more chemical reactions. Let's summarize: Lewis acids accept electron pairs, while Lewis bases donate!
Applications of Lewis Theory
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s look at how this theory applies in real-world scenarios. What reactions can you think of where this theory is particularly relevant?

Maybe in organic reactions where no hydrogen ion transfers occur?

Very good! In organic synthesis, many reactions that form bonds involve electron pair donation rather than just proton transfers. Remember, reactions of electron-deficient species are common.

Are there ways to identify if a substance is a Lewis acid or base?

Great question! Typically, look at their electron configuration. If a species can accept electrons or has a positive charge (like H+) or empty orbitals, it’s likely an acid. If it has lone pairs, it typically acts as a base. Let's engage with a memory aid: 'Lewis Loves Pairs' to remember that Lewis acids and bases work with electron pairs.
Reviewing Key Definitions
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

As we conclude today’s lesson, let’s recap the core concepts. Who can remember the primary definitions of Lewis acids and bases?

Lewis acids accept electron pairs, and Lewis bases donate electron pairs!

Excellent! And why is this important?

It broadens the understanding of acid-base reactions beyond just protons!

Exactly! Also remember the examples of BF3 and NH3 to remember how they interact. This completes our journey into the Lewis theory of acids and bases. Great job today!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section explains the Lewis theory, highlighting how Lewis acids may not always possess protons, using examples such as BF3 and NH3. It contrasts Lewis definitions with Brönsted-Lowry definitions while emphasizing their complementary nature in acid-base chemistry.
Detailed
Lewis Acids and Bases
G.N. Lewis (1923) expanded the definitions of acids and bases beyond those established by Arrhenius and Brönsted-Lowry. In this framework, an acid is defined as a species that accepts electron pairs, while a base is one that donates electron pairs. This conceptual shift allows for the categorization of many acid-base reactions where there is no H+ involved.
Key Examples
A classic example illustrating this concept is the reaction between the electron-deficient species boron trifluoride (BF3) and ammonia (NH3):
Reaction Example
$$
BF3 + :NH3 \rightarrow BF3:NH3
$$
In this reaction, BF3 acts as a Lewis acid by accepting a lone pair from the ammonia molecule, which acts as a Lewis base. This distinctive perspective encompasses compounds lacking protons that can still exhibit acidic behavior, such as AlCl3, Co3+, and Mg2+, which are all Lewis acids because they can accept electron pairs from bases like water and ammonia.
In contrast, substances such as H2O, NH3, and OH– serve as Lewis bases due to their ability to donate their electron pairs.
This definition is particularly useful in explaining several acid-base reactions that do not conform to traditional Arrhenius or Brönsted-Lowry theories, thereby enriching the understanding of chemical interactions.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Lewis Acids and Bases
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
G.N. Lewis in 1923 defined an acid as a species which accepts electron pair and base which donates an electron pair. As far as bases are concerned, there is not much difference between Brönsted-Lowry and Lewis concepts, as the base provides a lone pair in both the cases.
Detailed Explanation
G.N. Lewis introduced a broader perspective on acids and bases compared to earlier definitions. According to his definitions, a Lewis acid is any molecule or ion that can accept an electron pair, while a Lewis base is a molecule or ion that can donate an electron pair. This is different from the Arrhenius and Brönsted-Lowry definitions, which focus primarily on protons (H+). Lewis bases will still provide a lone pair of electrons regardless, but the key difference is that many Lewis acids do not have protons to donate.
Examples & Analogies
Think of Lewis acids as 'vacuum cleaners' that can suck up extra electrons from other molecules (the 'dust'), while Lewis bases are 'donors' that give away their extra electrons (like donating old toys). For instance, when ammonia (NH3) donates its lone pair to an electron-deficient molecule like boron trifluoride (BF3), BF3 acts as a Lewis acid, accepting the electron pair from NH3.
Example of Lewis Acid-Base Reaction
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
A typical example is reaction of electron deficient species BF3 with NH3. BF3 does not have a proton but still acts as an acid and reacts with NH3 by accepting its lone pair of electrons. The reaction can be represented by, BF3 + :NH3 → BF3:NH3.
Detailed Explanation
In the reaction between boron trifluoride (BF3) and ammonia (NH3), BF3 acts as a Lewis acid because it has an incomplete octet and can accept electrons. Conversely, NH3 donates its lone pair of electrons to BF3, acting as a Lewis base. This results in the formation of a coordinate covalent bond in the complex BF3:NH3, showcasing how Lewis acid-base reactions can occur without the transfer of protons.
Examples & Analogies
Consider this reaction as a scenario where BF3 is like a person asking for help to carry something heavy (its inability to complete its octet makes it 'weak'). NH3, having a strong desire to help, offers its hand (its lone pair) to BF3, thereby forming a team (the complex BF3:NH3) that is stronger together than either one alone.
Identification of Lewis Acids and Bases
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Electron deficient species like AlCl3, Co3+, Mg2+, etc. can act as Lewis acids while species like H2O, NH3, OH– etc. which can donate a pair of electrons, can act as Lewis bases.
Detailed Explanation
Certain elements or compounds can be classified as Lewis acids or bases based on their ability to accept or donate electrons. Lewis acids, like aluminum chloride (AlCl3) or metal cations such as cobalt (Co3+) and magnesium (Mg2+), can accept electron pairs due to their electron deficiency. On the other hand, water (H2O), ammonia (NH3), and hydroxide ions (OH–) act as Lewis bases because they can donate their electron pairs, aiding in various chemical reactions.
Examples & Analogies
Imagine a dance floor - the Lewis acids are those who are waiting for a partner (electron pairs) to join them for a dance, while the Lewis bases are eager dancers, ready to extend their hands (donate their electrons) to anyone in need of a partner. Thus, whenever a Lewis base encounters a Lewis acid, they form a harmonious pair and create a beautiful chemistry together.
Key Concepts
-
Lewis Acids: Electron pair acceptors.
-
Lewis Bases: Electron pair donors.
-
Electron-deficient species can act as Lewis acids.
-
Water can act as both an acid and a base depending on its reaction partner.
Examples & Applications
BF3 + NH3 → BF3:NH3 where BF3 is a Lewis acid that accepts a pair of electrons from NH3, a Lewis base.
In a reaction between AlCl3 and Cl–, AlCl3 behaves as a Lewis acid.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Lewis loves pairs, that’s how we’ll compare, acids accept, while bases repair!
Stories
Think of a bank where acids are like banks accepting deposits (electron pairs) while bases are the generous customers giving their deposits (electron pairs) away.
Memory Tools
Remember, ‘Lewis Leaves’ the door open for acids to 'accept' and bases to 'donate'.
Acronyms
A for Acceptor in Lewis acid; B for Base in Lewis base.
Flash Cards
Glossary
- Lewis Acid
A substance that can accept an electron pair from a Lewis base.
- Lewis Base
A substance that can donate an electron pair to a Lewis acid.
- Electron Pair
A pair of electrons that are found in the outer shell of an atom and can be shared or transferred to form bonds.
Reference links
Supplementary resources to enhance your learning experience.
