HOMOGENEOUS EQUILIBRIA
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to Homogeneous Equilibria
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we are going to explore homogeneous equilibria. Can someone tell me what it means when we say all reactants and products are in the same phase?

It means that they exist either all in gas form or all in a solution, right?

Exactly! This characteristic allows us to apply certain principles like the equilibrium constant. What do you think that is?

Is it a ratio that defines the relationship between the concentrations of reactants and products at equilibrium?

Spot on! In a reaction at equilibrium, Kc is defined as the ratio of the concentrations of products raised to their coefficients, divided by the reactants in the same way. Can anyone give me an example of an equation that might illustrate this?

How about the reaction for the synthesis of ammonia, N2 + 3H2 ⇌ 2NH3?

Great example! Here, Kc would be calculated as: Kc = [NH3]^2 / [N2][H2]^3. Now, remember, equilibrium doesn’t mean that the reaction has stopped. Can anyone explain what dynamic equilibrium means?

It means that even at equilibrium, the forward and reverse reactions are still occurring, but at the same rate.

Exactly right! Let’s lock in this idea of dynamic equilibrium as we move forward.
Le Chatelier’s Principle
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now that we understand Kc, let's discuss Le Chatelier's principle. Can anyone tell me what this principle states?

It states that if an external change is applied to a system at equilibrium, the system will adjust to counteract that change.

Correct! So, if we increase the concentration of a reactant, what would happen to the equilibrium?

The equilibrium would shift towards the products to minimize that change.

Yes! That shifting is crucial to maintaining equilibrium. What other factors do you think can affect equilibrium?

Changing both the temperature and pressure can affect it, right?

Absolutely! Temperature changes can shift equilibrium in endothermic or exothermic reactions. Let’s discuss how right now if we were to heat up our ammonia synthesis reaction, what shift would we expect?

It would shift to produce more reactants since it’s exothermic.

That's right! So, see how these principles tie together? Understanding these shifts helps chemists control reactions better.
Applications of Equilibrium Constants
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Next, let's talk about the applications of our knowledge of equilibrium constants. How might we determine if a reaction is at equilibrium?

By measuring the concentrations of the reactants and products.

Exactly! And by calculating Kc from these concentrations, we can compare it to our known value of K at that temperature. If Kc doesn’t match, then we know the system isn’t at equilibrium. What happens when you manipulate concentrations in a lab?

It directly impacts the equilibrium, forcing a shift to re-establish balance.

Right! This practical understanding allows chemists to maximize product yields in industrial processes. Let’s go over how Kp relates to Kc using the ideal gas equation.

Kp is Kc adjusted for pressure, especially for gas systems?

Exactly! And remember, Kp can be expressed as Kc times RT raised to the power of Δn. Can you recall what Δn signifies?

It’s the change in the number of moles of gases between products and reactants.

Perfect! This knowledge is highly valuable for systems involving gases.
Physical vs Chemical Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

In this final session, let’s differentiate between physical and chemical equilibria. How would you define physical equilibrium?

It’s a state where the physical processes, like melting or evaporation, are balanced.

Exactly! And what about chemical equilibrium?

That’s when the reactants and products are in a balanced state, with rates of forward and reverse reactions equal.

Well articulated! Can you think of examples of each type of equilibrium?

Phase changes like ice melting for physical and reactions like the synthesis of ammonia for chemical.

Great examples! Both types of equilibria demonstrate the fundamental principles we've learned today.

I'm glad we discussed this today! It gives me a clearer view of how equilibrium is important in chemistry.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
The section elaborates on the dynamics of heterogeneous equilibria, explaining how reactants and products in a single phase (gas or solution) reach equilibrium. Key concepts include the equilibrium constant, factors affecting equilibrium, and the relationship between Kp and Kc.
Detailed
Homogeneous Equilibria
In a homogeneous system, all reactants and products exist in the same phase, allowing for balanced reactions that reach equilibrium. This section explores the significance of chemical equilibria in various biological and environmental processes, citing examples like the role of hemoglobin in oxygen transport.
When considering a closed system, equilibrium is achieved when the rate of the forward reaction equals the rate of the reverse reaction. The shift in concentration for reactants and products is captured by the equilibrium constant (Kc), represented as:
Kc = [products]^[coefficients] / [reactants]^[coefficients].
These constants are dependent on temperature and can change with shifts in concentration and pressure, as outlined by Le Chatelier's principle. Thus, understanding the dynamic nature of equilibrium is essential for predicting the outcomes of chemical reactions. Factors like concentration changes, pressure variations, and temperature adjustments all influence the behavior of equilibria in a controlled environment.
The section further delineates between the equilibrium constants for gas reactions (Kp) and concentration-based reactions (Kc), establishing a formula that relates the two as: Kp = Kc(RT)^Δn for reactions involving gaseous species.
Youtube Videos









Audio Book
Dive deep into the subject with an immersive audiobook experience.
Definition of Homogeneous Equilibrium
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
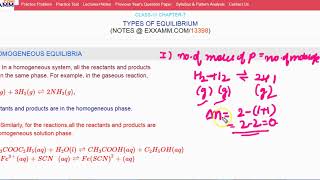
In a homogeneous system, all the reactants and products are in the same phase. For example, in the gaseous reaction, N2(g) + 3H2(g) 2NH3(g), reactants and products are in the homogeneous phase. Similarly, for the reactions, CH3COOC2H5 (aq) + H2O (l) CH3COOH (aq) + C2H5OH (aq) and Fe3+ (aq) + SCN–(aq) Fe(SCN)2+ (aq) all the reactants and products are in homogeneous solution phase. We shall now consider equilibrium constant for some homogeneous reactions.
Detailed Explanation
Homogeneous equilibrium refers to the state of equilibrium in reactions where all the chemicals involved (both reactants and products) exist in the same physical state, either as gases or in solution. This means that if the reaction involves gases, all species should be in the gaseous state; if in solution, they should all be in the aqueous phase. This is crucial because it allows us to easily manipulate and calculate concentrations, leading to consistent equilibrium constant expressions.
Examples & Analogies
Think of a smoothie: you take different fruits (reactants) and blend them into a uniform drink (products). Just like the fruits in the blender become a single homogeneous mixture, in a homogeneous equilibrium, the reactants and products are mixed together in the same phase, making it easier to analyze their properties and interactions.
Equilibrium Constant in Gaseous Systems
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
So far we have expressed equilibrium constant of the reactions in terms of molar concentration of the reactants and products, and used symbol, Kc for it. For reactions involving gases, however, it is usually more convenient to express the equilibrium constant in terms of partial pressure.
Detailed Explanation
In most cases, the equilibrium constant is defined in terms of the concentrations of the reactants and products. However, for gaseous reactions, using the partial pressures of the gases instead can be more practical. Partial pressure is the pressure that a particular gas in a mixture would exert if it occupied the entire volume by itself at the same temperature. When using partial pressures, we refer to the equilibrium constant as Kp. The relation between Kc and Kp can also be derived from the ideal gas law, emphasizing its usefulness in understanding reactions in gaseous states.
Examples & Analogies
Consider a balloon filled with air. The air pressure inside the balloon (the partial pressure of the air) can give you insights into how much gas is present at a certain volume and temperature. Similarly, in a reaction involving gases, understanding the partial pressures helps us predict how the system behaves at equilibrium.
Equilibrium Constant Expression
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
For reaction H2(g) + I2(g) 2HI(g), we can write either Kc = [HI(g)]^2 / [H2(g)][I2(g)] or Kp = (pHI)^2/(pH2)(pI2).
Detailed Explanation
The equilibrium constant expression quantitatively describes the relationship between the concentrations (or pressures) of reactants and products at equilibrium. For a general reaction, it shows that the concentration of products raised to the power of their coefficients in the balanced equation is equal to the concentration of reactants raised to the power of their coefficients. When dealing with gases, we can express this relationship using their partial pressures. This provides a mathematical method to calculate the state of the system when it's at equilibrium.
Examples & Analogies
Imagine a seesaw where weights on either side must balance out. The equilibrium expression is like the balance point of the seesaw where the weight on the product side (right) needs to balance with the weight on the reactant side (left). If one side is heavier, the seesaw tips, and the reaction shifts to try to reach balance again—this is similar to how reactions shift to maintain equilibrium.
Dynamic Nature of Equilibrium
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
At equilibrium, while the rates of the forward and reverse reactions are equal, the concentrations of the reactants and products remain constant, indicating a dynamic yet stable condition.
Detailed Explanation
Dynamic equilibrium is a state where the processes of reaction in both directions continue to occur, but there is no net change in the concentrations of the reactants and products. This means molecules are constantly converting from reactants to products and vice versa, creating a stable system where the concentrations of each substance remain the same over time. It's like a busy intersection where cars keep moving but the number of cars in any single lane stays constant due to continuous flow.
Examples & Analogies
Think of a dance floor where couples are constantly switching partners but the total number of couples remains the same. Even though movements are happening, the overall composition of couples dancing (like the equilibrium concentration of reactants and products) stays unchanged, capturing the essence of dynamic balance.
Factors Affecting Equilibrium Constant
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
An important feature of equilibrium constant is that it is only influenced by temperature. Changes in concentration or pressure do not change the value of Kc or Kp directly.
Detailed Explanation
The equilibrium constant for a reaction reveals the extent to which reactants can convert into products. While changing the concentration or pressure may shift the equilibrium position (favoring reactants or products), it does not alter the constant itself unless there is a change in temperature. The temperature affects the energy states of molecules, consequently impacting how favorably reactants can transform into products. This principle underpins much of chemical reaction dynamics.
Examples & Analogies
Consider a garden during the changing seasons. The types and number of plants (reactants and products) may change due to watering (adding concentration) or sunset (changes in conditions), but the overall type of ecosystem (the equilibrium constant) remains stable unless there’s a significant climate change (alteration in temperature).
Key Concepts
-
Homogeneous Equilibria: All reactants and products are in the same phase.
-
Dynamic Equilibrium: Forward and reverse reactions occur at equal rates.
-
Equilibrium Constants: Kc and Kp define the relationship between concentrations and partial pressures.
-
Le Chatelier's Principle: The equilibrium position shifts in response to changes in conditions.
-
Dynamism in Equilibrium: Even at equilibrium, reactions continue but the ratios remain constant.
Examples & Applications
The equilibrium constant for the reaction N2 + 3H2 ⇌ 2NH3 can be calculated to illustrate how Kc functions in practice.
Le Chatelier’s Principle can be demonstrated by adding reactants to an equilibrium mixture of H2 and I2 gases.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
For Kc high, products fly, for Kc low, reactants grow.
Stories
Imagine a seesaw: adding weight to one end forces a shift, just like adding reactants forces equilibrium to adapt.
Memory Tools
Remember 'R.E.A.C.T.' for the factors affecting equilibrium: Reactants, Energy, Additions, Changes, and Temperature.
Acronyms
Kc = Concentrations kept constant at Equilibrium.
Flash Cards
Glossary
- Homogeneous Equilibria
Equilibria involving all reactants and products in the same phase.
- Equilibrium Constant (Kc)
A numerical value that represents the concentrations of products to reactants at equilibrium for a reaction.
- Dynamic Equilibrium
A state in which the rates of forward and reverse reactions are equal, but both reactions are still occurring.
- Le Chatelier's Principle
A principle stating that a change in concentration, temperature, or pressure will shift the equilibrium position to counteract the change.
- Kp
The equilibrium constant expressed in terms of partial pressures of gaseous reactants and products.
Reference links
Supplementary resources to enhance your learning experience.
