Solid- liquid Equilibrium
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Understanding Equilibrium
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we'll learn about solid-liquid equilibrium. Can anyone tell me what equilibrium means in this context?

I think it means that the ice and water balance each other out!

Exactly! Equilibrium in this case signifies that the amount of ice and water remains constant. This is called static equilibrium. But there's more—it's actually dynamic. Let's dive deeper into that.

What do you mean by dynamic, Teacher?

Great question! When we say dynamic equilibrium, it means that both the melting of ice and the freezing of water are happening at the same time and at the same rate. This constant activity keeps the amounts of ice and water unchanged!
Molecular Activity
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Now, let’s discuss the molecular level interactions at the point where ice and water meet. Can anyone describe what happens there?

Are the molecules moving back and forth?

Yes! Ice molecules can escape into the water while water molecules collide with the ice and stick to it. This continuous movement is an essential characteristic of dynamic equilibrium.

Does that mean their temperatures have to match?

Exactly! The equilibrium occurs at a specific temperature and pressure. Do you remember the specific temperature for water and ice at equilibrium?

It's 273K!

That's correct! This is known as the normal melting point or freezing point of water.
Application of Knowledge
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Understanding solid-liquid equilibrium aids us in many practical situations. Can anyone give me an example?

Like how ice melts in drinks?

Exactly! When ice is added to a drink, it melts until it reaches a balance known as equilibrium with the liquid. How does this dynamic interplay benefit our drinks?

It keeps the drink cool!

Exactly! The continual melting and freezing keep the temperature stable until all the ice has melted. This concept can be applied in other fields too, such as food preservation and climate studies.

That makes sense now!
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
In a thermally insulated system at 273K and atmospheric pressure, ice and water maintain a dynamic equilibrium where the processes of melting and freezing occur simultaneously at equal rates. The conditions for this equilibrium are defined, emphasizing the normal melting and freezing points of pure substances.
Detailed
Solid-Liquid Equilibrium
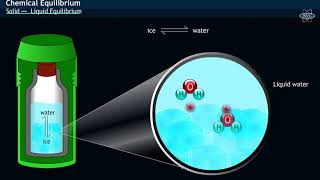
In this section, we explore the solid-liquid equilibrium using the example of ice and water in a perfectly insulated thermos flask at 273K and atmospheric pressure. At this temperature, the ice and water are in a stable equilibrium state where their masses remain constant over time. This equilibrium, however, is dynamic; it includes continuous molecular activity at the boundary where ice meets water. In this state, water molecules collide with the ice, causing some to adhere to its surface, while molecules from the ice transition into the liquid phase.
Key Features of the Equilibrium:
- Constant Mass: The total mass of ice and water does not change because the rates at which water melts into ice and ice melts into water are equal.
- Dynamic Nature: Despite the constancy in mass, there is a significant transfer of molecules between the phases.
- Specific Conditions: The equilibrium occurs at specific temperatures and pressures, explicitly detailing that at normal atmospheric pressure, the temperature where the solid and liquid phases coexist is termed the normal melting point or freezing point.
- Simultaneous Processes: The dynamic equilibrium can be understood as two opposing processes occurring concurrently at equal rates, which keeps the quantities of ice and water stable.
Youtube Videos






Audio Book
Dive deep into the subject with an immersive audiobook experience.
Equilibrium State of Ice and Water
Chapter 1 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Ice and water kept in a perfectly insulated thermos flask (no exchange of heat between its contents and the surroundings) at 273K and the atmospheric pressure are in equilibrium state and the system shows interesting characteristic features. We observe that the mass of ice and water do not change with time and the temperature remains constant.
Detailed Explanation
In a system where ice and water are in equilibrium at 273K, both phases coexist without any net change in mass. Although the temperature remains constant, this equilibrium is dynamic, meaning that while the overall mass doesn't change, there is continuous movement of molecules between solid ice and liquid water. Molecules from the liquid state can adhere to the ice, while some ice molecules can melt and enter the liquid phase. This balance results in a stable system.
Examples & Analogies
Think of a busy train station where trains (ice) and passengers (water) are constantly moving. While the number of trains and passengers at the station stays the same, there is always back-and-forth movement. Just like at the station, even though the mass of ice and water stays constant, there's an ongoing transfer of molecules between the two states.
Dynamic Equilibrium
Chapter 2 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
However, the equilibrium is not static. The intense activity can be noticed at the boundary between ice and water. Molecules from the liquid water collide against ice and adhere to it and some molecules of ice escape into liquid phase. There is no change of mass of ice and water, as the rates of transfer of molecules from ice into water and of reverse transfer from water into ice are equal at atmospheric pressure and 273 K.
Detailed Explanation
Dynamic equilibrium implies that even when overall amounts remain constant, continual exchanges are happening. At the boundary between ice and water, molecules from water phase are colliding with ice, sometimes sticking and causing it to gain mass, while other ice molecules melt and move into the liquid phase. Since these processes occur at the same rate, the net amounts remain unchanged, demonstrating dynamic activity.
Examples & Analogies
Consider a seesaw with two kids. When the weights on both sides are equal, the seesaw remains balanced, but both kids are actively moving up and down; they don’t stop moving. This is like dynamic equilibrium where constant action is occurring even while the overall scenario stays unchanged.
Conditions for Equilibrium
Chapter 3 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
It is obvious that ice and water are in equilibrium only at particular temperature and pressure. For any pure substance at atmospheric pressure, the temperature at which the solid and liquid phases are at equilibrium is called the normal melting point or normal freezing point of the substance.
Detailed Explanation
The establishment of equilibrium between solid and liquid phases (like ice and water) occurs at specific conditions: notably, a particular temperature and pressure. The normal melting point of a substance refers to this specific temperature under standard atmospheric pressure where ice exists in balance with liquid water.
Examples & Analogies
Imagine a dance floor where couples dance only in pairs. If one dancer leaves the floor, there will be no more pairs, and the dance stops. Similarly, without the right temperature and pressure, ice can no longer coexist with water in balance, just as couples need each other to keep dancing.
Characteristics of Dynamic Equilibrium
Chapter 4 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
The system here is in dynamic equilibrium and we can infer the following: (i) Both the opposing processes occur simultaneously. (ii) Both the processes occur at the same rate so that the amount of ice and water remains constant.
Detailed Explanation
In dynamic equilibrium, two opposing reactions (like melting of ice and freezing of water) happen at the same time, ensuring that their effects balance each other out. Therefore, even though individual molecules are transitioning between phases, the overall amounts of ice and water do not change.
Examples & Analogies
Think of an elevator in a tall building. People get on and off at various floors continuously, but if the number of people entering equals those exiting, then the total number of people in the lobby remains unchanged. Similarly, in dynamic equilibrium, the action of molecules moving between ice and water keeps their total amounts constant.
Summary of Solid-Liquid Equilibrium
Chapter 5 of 5
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
In summary, solid-liquid equilibrium is characterized by a system where the solid and liquid phases coexist at a specific temperature and pressure, maintaining a dynamic state where opposing processes occur at equal rates.
Detailed Explanation
This section on solid-liquid equilibrium explains how, at a constant temperature and pressure, ice and water can exist together without any net change in their amounts due to the ongoing processes of melting and freezing that occur at equal rates.
Examples & Analogies
Just like a balanced bank account where deposits and withdrawals occur at the same rate, solid-liquid equilibrium maintains constant levels of ice and water, allowing for a stable coexistence of both phases.
Key Concepts
-
Equilibrium: A stable state where conditions remain constant, often involving dynamic processes.
-
Molecular Activity: Continuous movement of molecules between solid and liquid phases that maintains equilibrium.
-
Normal Melting Point: The temperature at which a pure substance's solid and liquid phases coexist in equilibrium.
Examples & Applications
The process of ice melting in a drink until the drink reaches a stable temperature.
The phase transition of water to ice when the temperature drops below its normal melting point.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Ice and water, side by side, melting, freezing, cannot hide.
Stories
In a thermos, ice and water lived happily, swapping places in a dance of equilibrium.
Memory Tools
MFM - Melting Freezing Match: when melting and freezing rates are even, equilibrium is achieved.
Acronyms
MELT - Mass Equal at Liquid and solid temperature.
Flash Cards
Glossary
- Dynamic Equilibrium
A state where two opposing processes occur simultaneously at the same rate, resulting in no net change.
- Normal Melting Point
The temperature at which a pure substance changes from solid to liquid at atmospheric pressure.
- Mass Transfer
The movement of molecules between the solid and liquid phases, crucial in maintaining equilibrium.
Reference links
Supplementary resources to enhance your learning experience.
