Common Ion Effect in the Ionization of Acids and Base
Enroll to start learning
You’ve not yet enrolled in this course. Please enroll for free to listen to audio lessons, classroom podcasts and take practice test.
Interactive Audio Lesson
Listen to a student-teacher conversation explaining the topic in a relatable way.
Introduction to the Common Ion Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Today, we're discussing the common ion effect, which is an essential concept in understanding how ionic solutions behave. Can anyone explain what they think this effect refers to?

Is it about ions that frequently appear in different compounds?

That's close! The common ion effect specifically refers to how the solubility of an ionic compound is affected by the presence of a common ion from another source. For instance, if we add acetate ions to a solution containing acetic acid, it can impact the ionization of the acid.

So, adding something that shares an ion will change the balance?

Exactly! This is related to Le Chatelier's Principle. Can anyone summarize what that principle states?

It says a system at equilibrium will shift to counteract changes.

Correct! So, when we add a common ion, the equilibrium shifts, often reducing the ionization of the acid or base. Let's summarize the common ion effect before moving on.
Impact on Acid-Base Ionization
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

Let's delve deeper into how the common ion effect influences acid and base ionization. When we add acetate ions to a solution of acetic acid, how do you think this affects the concentration of H+ ions in the solution?

I think it would decrease the H+ concentration because it shifts the equilibrium to the left.

Right again! So, with more acetate ions, the equilibrium shifts to produce less H+, thus raising the pH. This concept is crucial in applications like buffering solutions. Anyone familiar with how buffers work?

Yes! Buffers help maintain a stable pH by using weak acids and their corresponding salts.

Yes! The common ion effect plays a significant role in this stability. Remember, when an acid's conjugate base is added, it mitigates changes in pH. Let's summarize that before we continue.
Applications of the Common Ion Effect
🔒 Unlock Audio Lesson
Sign up and enroll to listen to this audio lesson

The common ion effect has important applications, especially in chemistry and environmental science. For example, it can be used in the precipitation of salts. Can anyone think of examples where this might be applied?

Like when we want to remove specific ions from a solution?

Absolutely! By introducing a common ion, the desired ion can be precipitated and removed from the solution, improving purity. This is particularly valuable in water treatment processes. Can anyone summarize the significance of the common ion effect?

It helps control pH levels, assists in buffer solutions, and aids in the precipitation of salts.

And it's also critical in understanding how changes in ion concentration affect solubility.

Exactly! You all have a solid understanding of the material covered today. Let me recap the key points.
Introduction & Overview
Read summaries of the section's main ideas at different levels of detail.
Quick Overview
Standard
This section explores the common ion effect in the context of acids and bases, demonstrating how the addition of a common ion affects the equilibrium concentration of ions in solution. It highlights the implications for acid-base behavior and solubility, showing how the presence of a common ion can shift equilibrium according to Le Chatelier's Principle.
Detailed
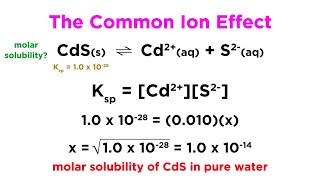
In the study of ionic equilibrium, the common ion effect is a critical concept that describes how the solubility of a sparingly soluble salt decreases in the presence of a solution containing a common ion. For example, when acetate ions are added to a solution of acetic acid, the equilibrium represented by the dissociation of acetic acid (HAc) into H+ and Ac– is disturbed. The addition of Ac– shifts the equilibrium to the left, resulting in a lower concentration of H+ ions. This principle is vital in understanding the ionization of weak acids and bases and is fundamentally explained by Le Chatelier's Principle, which states that a system at equilibrium will adjust to counteract any changes. The common ion effect is extensively utilized in analytical chemistry, especially in the precipitation of salts and the calculation of ionization constants.
Youtube Videos








Audio Book
Dive deep into the subject with an immersive audiobook experience.
Understanding the Common Ion Effect
Chapter 1 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Consider an example of acetic acid dissociation equilibrium represented as:
CH3COOH(aq) H+(aq) + CH3COO– (aq)
or HAc(aq) H+ (aq) + Ac– (aq)
Ka = [H+][Ac– ] / [HAc]
Detailed Explanation
The common ion effect occurs when an ion that is already present in a solution shifts the equilibrium position of a weak acid or base. In the case of acetic acid, when acetate ions (Ac–) are added to the solution, the equilibrium shifts to the left, favoring the formation of undissociated acetic acid (HAc). This results in a decrease in hydrogen ion concentration, which is crucial to understand the behavior of acids and bases in various scenarios.
Examples & Analogies
Imagine you have a party where guests are mingling (representing the dissociation of acetic acid). If you invite a group of friends who are already in a certain circle, they might pull more people into their group and reduce the number of mingling guests. This is similar to how adding acetate ions reduces the concentration of hydrogen ions in the solution.
Effects of Adding Common Ions
Chapter 2 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
Addition of acetate ions to an acetic acid solution results in decreasing the concentration of hydrogen ions, [H+]. Also, if H+ ions are added from an external source then the equilibrium moves in the direction of undissociated acetic acid i.e., in a direction of reducing the concentration of hydrogen ions, [H+].
Detailed Explanation
When you add acetate ions (Ac–) to the acetic acid solution, it disturbs the dissociation equilibrium and reduces the concentration of hydrogen ions [H+]. Similarly, if external hydrogen ions are introduced (for example, by adding a strong acid), the reaction shifts back towards the starting materials (HAc), further reducing the concentration of H+. This exemplifies the principle of Le Chatelier’s, which states that a system at equilibrium will adjust to counteract any changes applied to it.
Examples & Analogies
Think of a crowded marketplace where vendors (HAc) are selling apples (H+) and you introduce more vendors selling pears (Ac–). The increased presence of pears (Ac–) draws customers away from the apple vendors, resulting in fewer apple sales. If you suddenly provide free apples to customers, the original apple vendors will become overwhelmed, further reducing sales at their stalls.
Implications of the Common Ion Effect
Chapter 3 of 3
🔒 Unlock Audio Chapter
Sign up and enroll to access the full audio experience
Chapter Content
This phenomenon is an example of common ion effect. It can be defined as a shift in equilibrium on adding a substance that provides more of an ionic species already present in the dissociation equilibrium.
Detailed Explanation
The common ion effect is a crucial concept in understanding how adding a substance that contains an ion already present in the equilibrium can shift that equilibrium position. By introducing more of one species, you change the dynamics of the equilibrium, leading to a decrease in the solubility of a salt or the degree of dissociation of an acid or base.
Examples & Analogies
Consider a class project where students are required to produce a report. If you assign a few more students to the project team without clearing it with the class, it may disrupt the circle of ideas and lead to confusion. In chemistry, similarly, adding common ions can confuse the equilibrium, inducing a shift that can alter solubility or dissociation levels.
Key Concepts
-
Common Ion Effect: Refers to the reduction in solubility of an ionic compound when a common ion is present in the solution.
-
Le Chatelier's Principle: Describes how an equilibrium system responds to disturbances, such as changes in concentration, pressure, or temperature.
Examples & Applications
Adding sodium acetate to an acetic acid solution results in decreased ionization of the acid due to the common acetate ion.
The addition of chloride ions to a saturated solution of silver chloride leads to decreased solubility of the salt.
Memory Aids
Interactive tools to help you remember key concepts
Rhymes
Common ions in the mix, solubility they fix, adding them in, make compounds shrink, that's the common ion wink!
Acronyms
CIE
Common Ion Effect – Remember to ‘Counteract
Influence
Equilibrium’.
Stories
Imagine a party where guests brought the same dish; soon the dish becomes unappetizing, just like how common ions reduce solubility.
Memory Tools
Think ‘ICE’ for Initial, Change, Equilibrium when working with common ion effect problems.
Flash Cards
Glossary
- Common Ion Effect
A decrease in the solubility of an ionic compound due to the presence of a common ion.
- Le Chatelier's Principle
When a system at equilibrium is disturbed, it will shift in a direction to counteract the disturbance.
- Ionization
The process whereby an atom or molecule acquires a negative or positive charge by gaining or losing electrons.
- Equilibrium
A state in which the concentrations of reactants and products remain constant over time.
Reference links
Supplementary resources to enhance your learning experience.
